Abstract
Main conclusion
Various phenolic compounds of sorghum are effective in the management of abiotic stress (salt, nutrients) and biotic stress (caused by birds, fungi and aphids). The health and industrial application of phenolics is mainly contributed by inherent antioxidant and nutraceutical potential.
Abstract
In a natural environment, plant growth is affected by various biotic and abiotic stresses. In every ecosystem, the presence of a wide range of harmful biological agents (bacteria, fungi, nematodes, mites, and insects) and undesirable environmental factors (drought, salinity, heat, excessive or low rainfall, etc.) may cause a heavy loss in crop productivity. Being sessile during evolution, plants have evolved multiple defense mechanisms against various types of microbial pathogens and environmental stresses. A plant’s natural defense system produces some compounds named secondary metabolites, which include phenolics, terpenes, and nitrogen. The phenolic profile of grain sorghum, the least utilized staple crop, is unique, more diverse, and more abundant than in any other common cereal grain. It mainly contains phenolic acids, 3-deoxyanthocyanidins and condensed tannins. Sorghum polyphenols play a major role in plant defense against biotic and abiotic stresses and have many additional health benefits along with various industrial applications. The objective of this review is to discuss the phenolic compounds derived from grain sorghum and describe their role in plant defense, human health, and industrial applications.
Similar content being viewed by others
Introduction
Phenolics are the largest group of secondary metabolites in plants. They vary in shape from simpler aromatic rings to more complex ones, such as lignins. All these phenolic compounds originate from phenylalanine; therefore, they are also called phenylpropanoids. These phenols are synthesized by the phenylpropanoid pathway and are divided into several groups, such as phenolic acids, flavonoids, hydrolysable tannins, monolignols, stilbenes, and lignans, each with peculiar properties. Various phenolic compounds play an important role in the acclimatization of plants to unfavorable environmental conditions (Barcelos et al. 2016). The concentration of phenolic compounds in plant tissue is a good indicator for predicting the extent of abiotic stress tolerance in plants. It varies significantly in different plant species under an array of external factors, such as drought, heat, and cold. The growth and development, including seed germination, biomass accumulation, and metabolism of plants, are also influenced by plant phenolics. In this review, different types of sorghum phenolic compounds and their beneficial role in plant stress management, human health, and related industries have been discussed.
Sorghum grain and its nutritional composition
The C4 cereal sorghum grain is rich in polysaccharides (starch and non-starch), followed by proteins and lipids. The genetic characteristics of the cultivar, soil type, and environmental conditions during the season have a major impact on the content and composition of starch, i.e., the main polysaccharides in the grain. Sorghum has the lowest starch digestibility among cereals due to the strong association between the starch granules, proteins, and tannins (Mkandawire et al. 2013). Prolamins are major sorghum proteins with an average of 77–82% of the total proteins, and the remainder is albumins, globulins, and glutelins. The kafirins are the major prolamins of the sorghum and comprise three major classes: α-kafirins (66–84%), β-kafirins (8–13%), and γ-kafirins (9–21%) (Mokrane et al. 2010). Overall, the digestibility of sorghum proteins, especially after cooking, is lower than other cereals, such as wheat and maize. The main reason for the low digestibility of sorghum proteins is the resistance of kafirins to peptidase due to the formation of intramolecular disulfide bonds (Belton et al. 2006). Regarding the lipid profile of sorghum, it is 1.24–3.07 g/100 g of grain weight and mainly composed of unsaturated fatty acids. The primary fatty acids of sorghum are linoleic acid, oleic acid, palmitic acid, and linolenic acids. In most of the varieties of sorghum, the polyunsaturated fatty acids (PUFA) are higher in content than monounsaturated fatty acids (MUFA) (Mehmood et al. 2008). The sorghum genotypes have been studied elsewhere for various quality parameters (Kumari et al. 2016, 2017; Laxmi et al. 2019; Chakraborthy et al. 2020).
Sorghum is a source of various minerals, such as phosphorus, potassium, and zinc, whose contents vary according to cultivation. Although the content is known, the bioavailability of most of the minerals from sorghum is scarcely known. The bioavailability of zinc varies between 9.7% and 17.1%, and for iron, it ranges from 6.6 to 15.7%. Currently, efforts are being made worldwide to enhance the content and bioavailability of iron and zinc through biofortification, fortification, and genetic improvement of sorghum (Kruger et al. 2013). India’s first biofortified variety of sorghum, ICSR 14,001 with its higher iron and zinc, was developed by ICRISAT and released as ‘Parbhani Shakti’ for cultivation by Vasantrao Naik Marathwada Krishi Vidyapeeth (VNMKV), Maharastra.
Sorghum polyphenols
Phenolics are broadly distributed in the plant kingdom and are found abundantly as secondary metabolites of plants. Plant polyphenols have drawn increasing attention due to their potent applications in various fields (Dai and Mumper 2010). During the last decade, sorghum has attracted great attention from the food, feed, fodder and drug industries due to its unique phenolic profile, which helps it combat environmental stresses, such as biotic and abiotic stresses, along with its multifold human health benefits, including reducing oxidative stress and cancer prevention (Yang et al. 2009). The phenolic profile of grain sorghum is more diverse than those observed in other cereals, such as wheat, barley, rice, maize, rye, and oats. Phenolic acids, condensed tannins, flavonoids, stilbenes, and lignins are the major phenolics present in grain sorghum and are produced by the phenylpropanoid pathway. Among these phenolic acids, flavonoids (3-deoxyanthocyanidins), and condensed tannins are higher proportionally and biologically more active. The phenolics in sorghum grain are concentrated in the bran layer, and their content, concentration, and extractability vary greatly amongst sorghum varieties and genotypes (Ofosu et al. 2021). As the phenolic profile in sorghum is strongly associated with its bioactive properties, the knowledge of the phenolic structure, composition, location (that is, bran and kernel) and form of presence (that is, free and bound) in sorghum grain is crucial for the extraction method, material selection, and processing design, and thus, it is tailored for specific needs. Xiong et al. 2021 studied cellular antioxidant activity of sorghum phenolic extracts and reported that colored bran like brown and black sorghum has great potential to be used as a natural antioxidant for food and nutraceutical applications. The identification and development of phenolic compounds or extracts from plants have become a major area of health or related research (Dai and Mumper 2010). An overview of different types of sorghum phenolics is given in the following sections.
Phenolic acids
According to their structure, phenolic acids can be divided into two categories: hydroxybenzoic acid and hydroxycinnamic acid (Kumar and Goel 2019). The total concentration of phenolic acids in sorghum grain is in the range of 445–2850 μg/g (Girard and Awika 2018). These acids exhibit high antioxidant activity in vitro and thus have human health benefits (Kamath et al. 2004). The contents of primary phenolic acids based on studies of some sorghum varieties are provided in Table 1.
These phenolic acids occur in a bound state and have decreased bioavailability. They are not hydrolyzed by human digestive enzymes but are fermented by the colon's microbiota (Hole et al. 2012). The phenolic compounds of wines, fruits, and vegetables have a good bioavailability compared to the phenolic acids of cereals, including sorghum, because they are mostly bound to arabinoxylans chains or lignin (Abdel-Aal et al. 2012). Descriptive knowledge about techniques for improving the bioavailability of phenolic acids in sorghum is incipient. Therefore, microorganisms and grain processing play a key role in improving their bioavailability (Salazar-López et al. 2018). Cereal fermentation with specific probiotic strains and cooking processes can significantly increase the contents of free phenolic acids, thereby improving their bioavailability in sorghum (Saura-Calixto et al. 2010; N’Dri et al. 2013).
Tannin
Grain sorghum contains tannins with a high molecular weight and has a high degree of polymerization compared to other cereals, and they are the most investigated polyphenols in sorghum. The tannin content varies from 10.0 to 68.0 mg/g dry wt. in tannin sorghum compared to other cereals and pulses (Tannin-free sorghum at 0.5–3.8 mg/g, Finger millet at 3.6–13.1 mg/g, Buckwheat groats at 1.7 mg/g, and Cowpea at 1.8–2.9 mg/g) (Awika 2000). The concentration of tannin in sorghum cultivars, in relation to their color, varies significantly, for example, red and brown grain sorghums contain more bioactive compounds, such as tannins, which are considered beneficial to human health and are widely used in the beer and food industry (Eastin and Lee 2020). Based on tannin concentration and its extractability, sorghums can be classified into three types (Xiong et al. 2019). Type I sorghums with no pigmented testa thus have negligible or shallow levels of tannins (0–1.8 mg CAE/g). Type II sorghums have pigmented testa with moderate levels of tannins and are extractable with acidified methanol (6.4–15.5 mg CAE/g). Type III sorghums have pigmented testa with a high tannin concentration (11–50.2 mg CAE/g), are found mainly in the testa cell walls and the pericarp, and are extractable by methanol or acidified methanol (Dykes and Rooney 2006). In general, sorghums with pigmented testa have high levels of condensed tannin content, and Type III sorghums contain almost a ten times higher tannin concentration than other tannin-containing cereals (Girard and Awika 2018).
Despite the anti-nutritional effect, tannins have been extensively studied and used for human health-promoting capabilities because tannins are 15–30 times more effective than simple phenolics in radical scavenging ability. The functional benefits of sorghum are attributed mainly to oligomers, which have been extensively studied (Beecher 2004). The oligomers of tannins in foods contribute up to 19% of the antioxidant capacity of the diet, which benefits human health and promotes the prevention of non-communicable diseases due to immunomodulatory, anticancer, antioxidant, antiradical, anti-inflammatory, vasodilatory, cardio-protective, antithrombotic and anti-UV actions (Floegel et al. 2010).
The high tannin concentration in sorghum also offers an agronomical advantage over low tannin cultivars because the former protects the plants against pathogen and bird damage and can be grown in some under-developed regions of the world that have food security issues (Table 2) (Kil et al. 2009). The grain mold resistance of the genotype is significantly improved by a darker glume color, higher content of phenols, and the hardness of the seed (Audilakshmi et al. 1999). Recently, a known SNP (S4_62316425) in the TAN1 gene, a regulator of tannin accumulation in sorghum grain, was detected with a significant association with grain mold resistance (Nida et al. 2021), as the processing of phenolic acids improves the digestibility of tannins in sorghum. The processing of grain sorghum in dry heat (95 °C for 20 min and 121 °C for 30 min) can depolymerize the condensed tannins in sorghum (Barros et al. 2012), which can increase their bioavailability. Thermal processing is one strategy to increase the bioavailability of tannins with a minimum reduction in the content of these compounds. Thus, the functional potential of tannins-rich sorghum can be maintained or even increased. Furthermore, the reduction of polymeric tannins may boost the digestibility of starch and proteins, increasing the nutritional value of the grains. The depolymerization of tannins through other types of processing needs to be studied.
Flavonoids
Most flavonoids of the sorghum are located in the bran layers of the grain. The concentration of flavonoids is largely affected by the color and thickness of the pericarp and the presence of the testa (Dykes et al. 2011). Anthocyanins, flavones, and flavanones are major flavonoids that are present in the sorghum grain. Sorghum anthocyanins belong to the class of 3-deoxyanthocyanidins and correspond to up to 79% of the sorghum flavonoid content (Dykes and Rooney 2006). Due to the absence of a hydroxyl group at position C-3 in 3-deoxyanthocyanidins, they are more stable than other anthocyanins (Taleon et al. 2012).
The content of sorghum 3-deoxyanthocyanidins correlates with its color and antioxidant activity (Kayodé et al. 2011). Varieties with black pericarp and testa have 3–4 times more total 3-deoxyanthocyanidins (5.4–6.1 mg/g) than red and brown varieties (1.6–2.8 mg/g) (Awika et al. 2004). The total flavones of the sorghum vary from 0 to 386 mg/g (on average, 87 mg/g). The lowest content of flavonoids is found in white pericarp varieties, and the highest contents are observed in the lemon-yellow pericarp (474–1780 mg/g) (Dykes et al. 2011).
Stilbenes
Stilbenes belong to a small family of phenolic compounds derived from the phenylpropanoid pathway (Chong et al. 2009). They have numerous implications in plant disease resistance and human health. Stilbene content has a positive correlation with grain color and is present in smaller quantities in white-colored varieties. White sorghum contains traces of trans-piceid (up to 0.1 mg/kg) but lacks trans-resveratrol, whereas red sorghum has both (Bröhan et al. 2011). Stilbene compounds, a diverse group of natural defense phenolics, which are abundant in grapes, berries, sorghum, and conifer bark waste, may also confer a protective effect against aging-related diseases (Reinisalo et al. 2015).
Sorghum phenolics: applications in biotic and abiotic stress management
In the twenty-first century, to meet the food demand of the fast-growing human population, we need to enhance crop productivity and minimize crop losses. However, several biotic and abiotic stresses (insect pest attack, foliar and grain disease, drought, salinity, cold, heat, heavy metal toxicity, UV radiation, etc.), increased globalization and anthropogenic activities and induced climate changes are badly affecting a large proportion of arable land. These abiotic stresses affect plant growth and result in poor yield due to alteration in physiological and biochemical processes of plants (Wani et al. 2015). Plants exhibiting increased synthesis of polyphenols under biotic and abiotic stresses usually show better adaptability to limiting environments (Sharma et al. 2019).
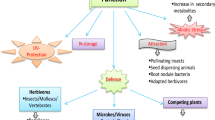
Sorghum’s ability to thrive under both biotic and abiotic stressors is mediated, in part, through the diverse families of secondary metabolites synthesized by a plant (Fig. 1). Sorghum possesses a variety of phytochemicals that are potentially helpful in overcoming the biotic and abiotic stresses in a plant. Various sorghum phenolic compounds, viz. phytoalexins (3-deoxyanthocyanidins) or allelochemicals (p-hydroxybenzoates, p-coumarates, and flavanols), play important roles in providing resistance for plants against biotic and abiotic stresses (Weir et al. 2004). Walling (2008) reported that some aphids are thought to have developed tolerance mechanisms against certain secondary metabolites. Interestingly, flavonoids have been suggested as candidate compounds that confer resistance to aphids in sorghum and other plant species (Kariyat et al. 2017). The genotypes accumulating higher levels of the cyanogenic glucoside (dhurrin) are resistant to aphids (Dreyer and Jones 1981) and the southwestern corn borer (Cheng et al. 2013). Many phenylpropanoids, phenolic acids, flavonoids, and condensed tannins have been implicated in plant resistance, with 3-deoxyanthocyanidins being the prominent ones (Deng and Lu 2017). Dicko et al. (2005) studied the relation between different phenolic compounds and biotic stresses (sooty stripe, sorghum midge, leaf anthracnose, striga and grain molds) and abiotic stress (lodging, drought resistance and photoperiod sensitivity) management and observed that sorghum varieties that have resistance to biotic and abiotic stresses had on average higher contents of 3-deoxyanthocyanidins (3-DAs), proanthocyanidins (PAs) and flavan-4-ols compared to susceptible varieties (Fig. 2). The contents of 3-DAs and PAs were suggested to be a good marker for resistance of sorghum to both biotic and abiotic stresses because these correlate with resistance to all stresses except for photoperiod sensitivity in grain sorghum.
Tannins: role in protection against bird damage
Bird damage is one of the most severe biotic constraints on crop production worldwide (De Mey et al. 2012; Anderson et al. 2013). Some cereal crops, such as wheat, rice, rye, sorghum, and millets, are more vulnerable to bird damage by lodging, pecking seeds and sucking the juice from immature seeds, preventing the full development of many grains and frequently encouraging mildews and other plant diseases around panicles (Tipton et al. 1970). Dixon et al. (2005) reported that the increased levels of condensed tannins (widely known as proanthocyanidins; PAs) in sorghum varieties also affect the sparrow feeding behavior. Based on GWAS analysis of a large-scale sorghum germplasm diversity panel, Xie and Xu (2019) revealed that Tannin1 encodes a WD40 protein functioning in the WD40/MYB/bHLH complex, which controls bird feeding behavior in sorghum. The study of sparrow feeding and sparrow attractant volatile assays confirmed the anti-feedant and anti-attractant functions of differentially accumulated metabolites on bird behavior. Bird-preference accessions possess a variety of aromatic and fatty acid-derived volatile accumulation at significantly higher levels.
Deoxyanthocyanidins: effectiveness against anthracnose fungus
In Sorghum, a group of phytoalexins is induced at the infection site by Colletotrichum sublineolum, the anthracnose fungus. These compounds, classified as 3-deoxyanthocyanidins, have structural similarities to the precursors of phlobaphenes. 3-Deoxyanthocyanidins were detected as major flavonoids in black sorghum grains (Taleon et al. 2012). The contribution of flavonoid phytoalexins in resistance against Colletotrichum sublineolum in sorghum has been investigated by comparing the response of several sorghum cultivars that differentially produce 3-deoxyanthocyanidins (Basavaraju et al. 2009). Loeh et al. (1999) reported that sorghum responds to the invasion of both pathogenic and nonpathogenic fungi by the induction of 3- deoxyanthocyanidin phytoalexins. Ibraheem et al. (2010) carried out an experiment by using yellow seed sorghum to study the effect of flavonoids on anthracnose leaf blight. It was reported that sorghum yellow seed 1 (y1) encodes a MYB transcription factor, which regulates phlobaphenes biosynthesis and its near-isogenic lines, but having loss-of-function alleles of y1 means that it is not able to accumulate phlobaphenes. Molecular characterization of the two null y1 alleles shows a partial internal deletion in the y1 sequence. These null alleles, designated as y1-ww1 and y1-ww4, do not accumulate 3-deoxyanthocyanidins when challenged with the nonpathogenic fungus Cochliobolus heterostrophus.
Furthermore, compared to the wild-type allele, both y1-ww1 and y1-ww4 show greater susceptibility to the pathogenic fungus C. sublineolum. In fungal-inoculated wild-type seedlings, y1 and its target flavonoid structural genes were coordinately expressed. However, in y1-ww1 and y1-ww4 seedlings, where y1 was not expressed, steady-state transcripts of its target genes were not detected. Co-segregation analysis showed that the functional y1 gene is genetically linked with resistance to C. sublineolum. In conclusion, a significant reduction in ALB disease symptoms was reported with a higher accumulation of known 3-deoxyanthocyanidins in sorghum plants carrying a functional y1 allele in response to infection by the anthracnose fungus C. sublineolum. In Sorghum bicolor metabolomic analysis of defense-related reprogramming in response to Colletotrichum sublineolum infection, it also revealed a functional metabolic web of phenylpropanoid and flavonoid pathways (Tugizimana et al. 2019).
Deoxyanthocyanidins: defense against corn leaf aphid
Sorghum is also a potential host to more than 150 insect pests with aphids being a major group of them (Sharma 1993). Almost four species of aphids feed on sorghum and corn leaf aphid (CLA) Rhopalosiphum maidis, and Fitch (Hemiptera, Aphididae) is the major one among them (Young and Teetes 1977). To defend against any damaging pests, plants have evolved a specific defense mechanism that is mainly classified into physical and chemical defenses. Among the physical defenses, leaf trichomes and epicuticular wax have been suggested to play a significant role against many herbivorous species, including aphids (Eigenbrode and Espelie 1995; Kariyat et al. 2017). Insect herbivory elicits complex counter defense responses from plants, including the biosynthesis of toxic secondary metabolites that act as chemical defense against particular infections, such as glycosides, alkaloids, benzoxazinoids, glucosinolates, and flavonoids (Betsiashvili et al. 2015). For example, vanillic and aconitic acids have been found to have antifeedant properties, and sorghum genotypes with higher polyphenol contents are less preferred by aphids (Mote and Shahane 1993). In sorghum, the y1-regulated flavonoid pathways have resulted in deleterious effects on aphids, resulting in defense against corn leaf aphid (Kariyat et al. 2017).
Phenolics: effect on grain mold
‘Grain mold’ is a significant biotic stress affecting the production, marketing, and productivity of grain sorghum. The term is used to describe the diseased appearance of sorghum grain resulting from infection of one or more pathogenic or saprophytic fungus. Funguses of more than 40 genera are associated with sorghum grain (Williams and Rao 1981). Most are restricted to the pericarp, but penetration to endosperm occurs if the mature grain is exposed to high humidity for a longer period at maturity. Audilakshmi et al. (1999) studied sorghum genotypes for various morphological and biochemical traits and their contribution to resistance for grain mold. Highly significant correlations between grain mold and seed hardness, seed phenolics content in acid methanol extract, and glume color revealed that they strongly affected the grain mold response. Harder grain, higher levels of seed phenols, and darker glumes contributed to grain mold resistance. Weaker and less consistent correlations were observed between grain mold and seed color, seed flavan-4-ol content, glume phenol, flavan-4-ol contents, and glume cover, indicating the relatively lower effect of these traits on grain mold response. It has been suggested that combinations of several attributes are required to achieve efficient resistance (Audilakshmi et al. 1999). Esele (1993) reported that a pigmented testa, where condensed tannins are present, is the most critical trait for conferring grain mold resistance. Red pericarp containing flavan-4-ol also plays a role in mold resistance but is not as effective as the pigmented testa. However, the combination of both provides additive effects on resistance. Melake-Berhan et al. (1996) also reported the same results, highlighting the correlation between tannin and flavan-4-ol with resistance in colored pericarp sorghums with pigmented testa. Not all red pericarp needs to be resistant to grain mold.
Flavonoids and their role in salt stress management in sweet sorghum
Abiotic stresses affect crop production and productivity worldwide. Plants have developed specific defense mechanisms against environmental stresses by altering the gene expression pattern, leading to the regulation of specific metabolic and defensive pathways. Sorghum is an essential crop in regions that are mainly irrigated by salty water. Sweet sorghum is a variant of common grain sorghum and is relatively more adapted to marginal growing conditions. Some phenolics, like anthocyanin and tannins, have a high antioxidant capacity and help in plant defense naturally against abiotic stresses, pests, and disease damage (Dempsey et al. 2011).
Meng et al. (2015) reported that flavonoids have critical physiological roles in plants; their accumulation is induced by abiotic stresses and is a hallmark of plant stress. In addition, it has been observed that salt-tolerant species often accumulate more flavonoids than salt-sensitive species, which suggest a relationship between flavonoid biosynthesis and salt stress resistance (Liu and Godwin 2012). The high flavonoid contents may have contributed to elevating the antioxidant activity of the plant tissues under stress. The flavonoid biosynthesis pathway played an essential role in the high salt tolerance in the sweet sorghum landraces, and six genes involved in the flavonoid biosynthesis pathway to tannins and anthocyanins from phenylalanine have been identified in the sweet sorghum landraces. Moreover, their expression was observed to be significantly different from that in grain sorghum, based on RNA-Seq (Genzeng et al. 2019). The study revealed that the accumulation of tannin positively relates to the sorghum salt-resistance and flavonoids biosynthesis, which plays a vital role in the sweet sorghum capacity for salt tolerance.
Phenolics and their impact on nutrient uptake in sorghum
Despite their role in biotic and abiotic stress management, phenolics also improve nutrient uptake through chelation of metallic ions, enhanced active absorption sites, and soil porosity which accelerate the mobilization of elements, such as calcium (Ca), magnesium (Mg), potassium (K), zinc (Zn), iron (Fe), and manganese (Mn) (Seneviratne and Jayasinghearachchi 2003). Sorghum is a rich source of flavonoids, such as flavonols, flavonones, flavons, and anthocyanins, which are particularly abundant in red and black sorghum grain (Dicko et al. 2005) but rare or absent in other plants (Awika et al. 2004). Some workers have reported that high plant density and intercropping practices reduced insect pest infestation in cowpea (Makoi et al. 2010). This was probably due to the excessive accumulation of phenolic compounds in plants growing in such systems.
Musa et al. (2011) studied sorghum–cowpea intercropping under treatment with chemical and bio fertilization, leading to enhanced critical macro and micronutrients (Ca, Mg, Cu, Mn, and Fe) of sorghum seeds. Because both cowpea and sorghum are the staple food in many of the semi-arid tropical regions, growing them in mixed culture may be the main source of natural antioxidants, and these types of practices must be tried in these areas. Although several studies have shown that stress affects the release of these compounds, further studies are required to assess the effects of flavonoid and anthocyanin compounds in the control of pests (insects, diseases, and weeds) in mixed culture systems.
Sorghum phenolic compounds: potential human health applications
Currently, consumers think about their health, healthy living, and health food even when it is at a high cost (Vyas et al. 2018; Chaudhary et al. 2021). Sorghum is a nutricereal that is composed of starch, proteins, unsaturated lipids, and some minerals and vitamins. Most grain sorghum varieties are a rich source of phenolic compounds and bioactive compounds, especially 3-deoxyanthocyanidins and tannins, which have a great health impact on human gut microbiota and reduce parameters related to obesity, oxidative stress, inflammation, diabetes, dyslipidemia, cancer and hypertension (de Morais Cardoso et al. 2017). In addition to direct antioxidant effects, the sorghum phenolic compounds also induce endogenous detoxifying enzymes (phase II enzymes) that are responsible for converting the harmful reactive oxygen or nitrogen species into nontoxic compounds, thus indirectly enhancing the human body defense mechanism against oxidative stress (Awika et al. 2009; González-Montilla et al. 2012).
Polyphenols against cancer
Most cancers originate from DNA damage caused by carcinogenic agents, such as toxins and mutagenics, that make up reactive intermediates, such as reactive oxygen species (ROS), reactive nitrogen species (RNS), and other reactive electrophilic metabolites (Sharma and Verslues 2010). The carcinogen rate in humans is strongly dependent on the activities of phase I (cytochrome P-450) and II enzyme systems, which also remove endogenous and environmental carcinogens (Takabe et al. 2006).
3-Deoxyanthocyanidins, a sorghum phenolic compound, have a strong influence on the phase II enzyme activity, especially on the enzyme NADH: quinone oxidoreductase (NQO) activity. 3-Deoxyanthocyanidins are strong NQO inducers. Both 3-deoxyanthocyanidin standards and 3-deoxyanthocyanidin-rich sorghum extract have been reported to increase the NQO activity in some cancer cells in particular. The inducing capacity of 3-deoxyanthocyanidins on the phase II enzyme varied greatly with their structure and substitution, such as methoxylated substitution at the C-5 and C-7 positions, such as with 7-methoxyapigeninidin and 5,7-dimethoxyapigeninidin, and can significantly enhance the inducing effect on the NQO activity (Yang et al. 2009). Because black and red sorghums are rich in 3-deoxyanthocyanidins, they have strong inducing effects on NQO activity. Surprisingly, white pericarp sorghums with low 3-deoxyanthocyanidin content have significant inducing effects on the NQO, indicating the possible role of other bioactive compounds that must also be investigated. However, overall epidemiological evidence has suggested that sorghum has anti-carcinogenic properties when consumed regularly in the diet (Jideani et al. 2014).
Polyphenols against dyslipidemia and cardiovascular disease
Dyslipidemia may be defined as increased levels of serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), or decreased serum high-density lipoprotein cholesterol (HDL-C) concentration. Dyslipidemia is an established risk factor for cardiovascular disease (CVD). Various epidemiological data indicate that whole grain consumption significantly lowers mortality from CVD (Anderson 2003). Animal studies also suggest that sorghum consumption promotes cardiovascular health better than other cereals. Klopfenstein and Owen (1981) reported a cholesterol-lowering effect of low-tannin sorghum grain when fed to guinea pigs at 58% of the diet. This effect was more significant than that produced by wheat, rolled oats, or pearl millet. In vitro and animal studies have shown that the lipidic and phenolic fractions from sorghum modulate parameters are related to dyslipidemia and the risk of cardiovascular disease (de Morais Cardoso et al. 2017). These benefits result from the action of phytosterols, policosanols, and other phenolics of sorghum, which may modulate absorption, excretion, and synthesis of cholesterol.
Diet supplementation with sorghum lipids reduced the hepatic and plasma cholesterol of normolipidemic hamsters (Hoi et al. 2009). The phytosterols are one of the major bioactive compounds from the sorghum lipid fraction that can inhibit cholesterol absorption. The phytosterols in the cereal brans are believed to contribute beneficial effects. Other components of the whole grains, including polyphenols and fiber, also play a role in CVD prevention. Sorghum is a significant source of phytosterols and policosanols (Singh et al. 2003). The benefits of sorghum to cardiovascular health may not be limited to positive effects on cholesterol. Lee and Pan (2003) demonstrated that dietary tannin–sorghum distillery residues inhibited 63–97% of hemoglobin-catalyzed oxidation of linoleic acid in cultured mullet fish compared to that in soybean (13%) and rice bran (78%), respectively.
Overcoming oxidative stress using phenolic compounds
The chronic and excessive production of free radicals in the human body is crucial in the development of non-communicable diseases (Lee et al. 2011). The activity of components isolated from sorghum against oxidative stress has been demonstrated in vitro by various workers. Moraes et al. (2012) reported that extracts from black or red sorghum, when used, produce functional benefits attributed to the phenolic compounds. Phenolic compounds isolated from sorghum regulate the expression of phase II enzymes, which play an important role in modulating the defense system against oxidative stress by continuously converting highly reactive electrophilic species (RES) into nontoxic and extractable metabolites (González-Montilla et al. 2012). Varieties of black sorghum may exert greater effects on NQO due to its rich profile and high content of 3-deoxyanthocyanidins (Devi et al. 2011).
Sorghum is a rich source of other phytochemicals, pigmented or not, that acts synergistically with 3-deoxyanthocyanidins and produce high inducer activity. The effects of sorghum on oxidative stress in vivo are not well known. The superoxide dismutase activity (SOD) increased in normolipidemic rats fed with black sorghum bran (rich in 3-deoxyanthocyanidins) has been reported (Lewis et al. 2008). This increase appears to be strictly related to the action of 3-deoxyanthocyanidins present in the bran. Furthermore, white (rich in phenolic acids), brown (rich in tannins), or black (rich in 3-deoxyanthocyanidins) sorghum brans suppressed the glutathione peroxidase activity (GPx). However, the normolipidemic animals fed with whole red sorghum had lower concentrations of thiobarbituric acid reactive substances (TBARS) in their livers (Moraes et al. 2012).
Anti-obesity and anti-inflammatory effects of phenolics
Obesity is a pandemic correlated with various non-communicable diseases and characterized by chronic low-grade inflammation. Adipocytes and obesity play an essential role in inflammatory mediators that signal this process. The discovery that obesity itself results in an inflammatory state in metabolic tissues opened a research field that examines the inflammatory mechanisms in obesity (Greenberg and Obin 2006). This unique understanding allows a more precise understanding of the role of adipocytes in health and obesity and about how inflammatory mediators that act as signaling molecules in this process (Gregor and Hotamisligil 2011). Sorghum as a whole grain is an excellent food for people with obesity because sorghum endosperm contains high levels of resistance and relatively low starch digestibility (Barros et al. 2012).
A study on rats, pigs, rabbits, and poultry suggested that tannin-rich sorghum reduces undesirable weight gain in obesity in humans (Muriu et al. 2002). Barros et al. (2013) demonstrated that sorghum polymeric tannins naturally modify starch by interacting strongly with amylose and form resistant starch. Resistant starch cannot be digested in the small intestine and thus reaches the large intestine, delivering the health benefits of dietary fiber (Sánchez-Zapata et al. 2015). Furthermore, sorghum tannins can inhibit starch digestion by inhibiting saccharase and amylase enzymes (Mkandawire et al. 2013). In another study, tannin-rich sorghums were found to be more effective than those rich in 3-deoxyanthocyanidins in inhibiting hyaluronidase, a vital enzyme associated with inflammation (Bralley et al. 2008).
Diabetes: hypoglycemic effect of phenolics
The commonly known forms of diabetes are T1DM, T2DM, and gestational diabetes (GD). Diabetes becomes a highly challenging health problem and is progressively prevalent globally with an estimated 1.5 million deaths per year (Ogurtsova et al. 2017). India has the world's second-largest number of people with diabetes after China (Wedick et al. 2015). This is a lifelong condition characterized by hyperglycemia in which the body is unable to secrete enough insulin. After a meal, a diabetic patient's glucose level rises intensely and prompts a fall down as the body is unable to stock the extra glucose for later use. Kam et al. (2016) reported that the use of gluten-free whole grains, such as sorghum quinoa, buckwheat, and minor millets might maintain the role of beta cells. Kim and Park (2012) have reported from animal studies that phenolic extracts of sorghum modulate glucose metabolism in animals due to the action of the phenolic compounds and exhibit a hypoglycemic effect similar to glibenclamida, an antidiabetic medication used in their control group.
Industrial applications of sorghum phenolic compounds
Currently, due to the adverse environmental impacts on human health and growing consumer awareness for healthy eating, there has been a great demand for foods or food ingredients that have a positive health impact. Thus, sorghum has recently attracted much attention in developed countries due to its high nutritional value and may enhance rapidly after this COVID-19 pandemic. Due to the diverse phenolic profile of sorghum and its diverse role in the food industry, its industrial application is discussed below.
Sorghum phenols as nutraceuticals
The use of sorghum phenolic compounds, especially tannins, for the development of functional foods and nutraceuticals is an innovative idea. First, Links et al. (2015) developed a nutraceutical by encapsulating sorghum-condensed tannins into kafirin microparticles that can withstand gastric digestion and have shown good anti-hyperglycemic effects both in vitro and in vivo (Links et al. 2015). Condensed tannins are strong gluten strengtheners, especially those with a large molecular weight and a high degree of polymerization, which are capable of forming extensive cross-linking with gluten proteins. Sorghum-condensed tannins have significantly increased dough and better viscosity and stability, thus improving food structural stability and quality. Sorghum-condensed tannin could be used as a natural ingredient to enhance the quality of gluten and enhance its functionality, suggesting its potential as a multifunctional ingredient in the food and biomedical industry (Girard et al. 2019).
Antioxidative preservation of food products using sorghum bran
Apart from the whole sorghum grain, sorghum bran also has a huge potential in the food industry. Sorghum bran is a high-value functional ingredient (Dykes 2019). The bran can be easily obtained by grain decortications and then used as a natural colorant and antioxidant preservative in food products to improve food quality and preservation. For instance, Luckemeyer et al. (2016) reported that the addition of 0.25–0.75% high-tannin sorghum bran to meat products, such as pre-cooked pork and turkey patties, was said to prevent lipid oxidation during storage without compromising the meat sensory flavor attributes. Similarly, Cabral et al. (2019) also noted that the addition of 0.5% high-tannin sorghum bran to pork pizza topping and dark chicken meat reduces lipid oxidation and rancid flavor. Although the addition of sorghum bran to meat products may also lead to a darker color and sorghum flavor, it does not necessarily indicate a poor meat quality or low consumer acceptance. Natural ingredients to improve food quality, safety, and health function while maintaining the sensory quality could be novel areas for future research.
Production of gluten-free beers/beverages for Celiac people
Sorghum provides the opportunity of producing gluten-free beers/beverages for celiac patients because it is a gluten-free cereal. Beer made of white sorghum has more than two times higher phenolic contents than barley beer, which contributes to its high antioxidant activity, and this beer also contains significant amounts of γ-aminobutyric acids with potential antihypertensive activity; it also has α-glucosidase inhibitory activity and low ethanol content. Consumption of this beer could promote human health if consumed in moderation by Celiac patients (Garzón et al. 2019).
Gluten-free cookies and biscuits for diabetics
Sorghum can be used to make gluten-free healthy snacks, such as cookies and biscuits for diabetics. Cookies made from tannin sorghum grain have been shown to have high phenolic contents and antioxidant activity, especially those with an antioxidant activity up to 20 times higher than wheat cookies (Chiremba et al. 2009). However, tannin sorghum cookies have low sensory acceptance despite their high antioxidant activity and great health properties. Thus, the production of nontannin sorghum cookies has great potential for commercialization and large-scale production. They have similar sensory acceptance as wheat cookies, with the phenolic contents and antioxidant activity being slightly lower than tannin sorghum cookies (Chiremba et al. 2009). Biscuits made of sorghum have been shown to reduce oxidative stress and inflammation and improve the glycemic response in people. It is an ideal alternative snack for people with obesity and diabetes (Stefoska-Needham et al. 2017).
Potential animal feed additive
Sorghum is a multipurpose crop and has a high demand as a fodder crop, especially in the kharif season. It also has great potential as an animal feed additive, which may improve animal health and production. Sorghum distillers’ grain, an industrial by-product from the ethanol production unit, is a cheap material used as an additive in pig and rabbit feeds. It is rich in immune activators resulting from fermentation that enhances immunity and improves animal health (Pomerenke et al. 2010).
Conclusion and future prospective
Modern genetic engineering and breeding tools provide exciting opportunities to develop sorghum with desirable nutritional and phenolic profiles while maintaining good agronomic performance and yield. This could be a fruitful area for further research under rapidly changing climatic conditions. It has been shown that through mutagenesis-assisted breeding, the biosynthesis of phenolic compounds can be enhanced in sorghum. A sorghum mutagenesis variant, RED for GREEN, which can significantly increase the 3-deoxyanthocyanidins, condensed tannins, and total phenolic contents in sorghum leaf, has been identified (Petti et al. 2015). Advances have also been made in breeding sorghum (germplasms ATx3363 and BTx3363) with high levels of 3-deoxyanthocyanidins in the grain pericarp and satisfying grain yield (Dykes et al. 2013).
It may be concluded that various phenolic compounds from sorghum grain play a great role in overcoming biotic and abiotic stresses, such as insect pest attacks, drought, heat, and salinity. Additionally, sorghum brans can be used to fortify bread, cookies, and other snacks to improve their phytonutrient content, dietary fibers, and sensory properties, resulting in a positive effect on human health. A major limitation for their effect is their low bioavailability, which in turn depends on cultivars. Many researchers worldwide are working on a better understanding of the phenolic profile of sorghum and its specific role in overcoming biotic and abiotic stresses, which is an urgent need because of the ever-changing climatic conditions. The focus must be on finding new extraction methods to increase their bioavailability in plants and humans. Sorghum, currently consumed in developing and underdeveloped countries but one-day it will be preferred in developed countries also due to its high bioactive compound concentration which is having a beneficial impact on both plant and human health.
Author contribution statement
PK, RK and SKP conceived and designed the manuscript theme. PK and VK wrote the manuscript. All authors read, edited and approved the manuscript.
Change history
12 October 2021
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00425-021-03748-4
References
Abdel-Aal ESM, Choo TM, Dhillon S, Rabalski I (2012) Free and bound phenolic acids and total phenolics in black, blue, and yellow barley and their contribution to free radical scavenging capacity. Cereal Chem 89:198–204. https://doi.org/10.1094/CCHEM-10-11-0116
Afify AEMMR, El-Beltagi HS, El-Salam SMA, Omran AA (2012) Effect of soaking, cooking, germination and fermentation processing on proximate analysis and mineral content of three white sorghum varieties (Sorghum bicolor L. moench). Not Bot Horti Agrobot Cluj-Napoca 40:92–98. https://doi.org/10.15835/nbha4027930
Anderson JW (2003) Whole grains protect against atherosclerotic cardiovascular disease. Proc Nutr Soc 62:135–142. https://doi.org/10.1079/pns2002222
Anderson A, Lindell CA, Moxcey KM et al (2013) Bird damage to select fruit crops: the cost of damage and the benefits of control in five states. Crop Prot 52:103–109. https://doi.org/10.1016/j.cropro.2013.05.019
Audilakshmi S, Stenhouse JW, Reddy TP, Prasad MVR (1999) Grain mould resistance and associated characters of sorghum genotypes. Euphytica 107:91–103. https://doi.org/10.1023/A:1026410913896
Awika JM, Rooney LW, Waniska RD (2004) Properties of 3-deoxyanthocyanins from sorghum. J Agric Food Chem 52:4388–4394. https://doi.org/10.1021/jf049653f
Awika JM, Yang L, Browning JD, Faraj A (2009) Comparative antioxidant, antiproliferative and phase II enzyme inducing potential of sorghum (Sorghum bicolor) varieties. LWT-Food Sci Technol 42:1041–1046
Awika J (2000) Sorghum phenols as antioxidants. Doctoral dissertation, Texas A&M University
Barcelos CA, Maeda RN, Santa Anna LMM, Pereira N (2016) Sweet sorghum as a whole-crop feedstock for ethanol production. Biomass Bioenerg 94:46–56. https://doi.org/10.1016/j.biombioe.2016.08.012
Barros F, Awika JM, Rooney LW (2012) Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J Agric Food Chem 60:11609–11617. https://doi.org/10.1021/jf3034539
Barros LAC, Cardoso De Aguiar HJA, Mariano CDSF, et al (2013) Cytogenetic characterization of the ant Trachymyrmex fuscus Emery, 1934 (Formicidae: Myrmicinae: Attini) with the description of a chromosomal polymorphism. In: Annales de la Societe Entomologique de France. Taylor & Francis, pp 367–373
Basavaraju P, Shetty NP, Shetty SH et al (2009) Infection biology and defence responses in sorghum against lletotrichum sublineolum. J Appl Microbiol 107:404–415. https://doi.org/10.1111/j.1365-2672.2009.04234.x
Beecher GR (2004) Proanthocyanidins: biological activities associated with human health. Pharm Biol 42:2–20. https://doi.org/10.1080/13880200490893474
Belton PS, Delgadillo I, Halford NG, Shewry PR (2006) Kafirin structure and functionality. J Cereal Sci 44:272–286. https://doi.org/10.1016/j.jcs.2006.05.004
Betsiashvili M, Ahern KR, Jander G (2015) Additive effects of two quantitative trait loci that confer Rhopalosiphum maidis (corn leaf aphid) resistance in maize inbred line Mo17. J Exp Bot 66:571–578. https://doi.org/10.1093/jxb/eru379
Bralley E, Greenspan P, Hargrove JL, Hartle DK (2008) Inhibition of hyaluronidase activity by select sorghum brans. J Med Food 11:307–312. https://doi.org/10.1089/jmf.2007.547
Bröhan M, Jerkovic V, Collin S (2011) Potentiality of red sorghum for producing stilbenoid-enriched beers with high antioxidant activity. J Agric Food Chem 59:4088–4094. https://doi.org/10.1021/jf1047755
Cabral JS, Whittaker RJ, Wiegand K, Kreft H (2019) Assessing predicted isolation effects from the general dynamic model of island biogeography with an eco-evolutionary model for plants. J Biogeogr 46:1569–1581. https://doi.org/10.1111/jbi.13603
Chakraborthy I, Kumari P, Pahuja SK et al (2020) Elucidation of combining ability and fodder potential of sorghum hybrids. Forage Res 46:132–140
Chaudhary N, Kumar V, Sangwan P et al (2021) Personalized nutrition and -omics. Compr Foodomics. https://doi.org/10.1016/B978-0-08-100596-5.22880-1
Cheng WN, Lei JX, Rooney WL et al (2013) High basal defense gene expression determines sorghum resistance to the whorl-feeding insect southwestern corn borer. Insect Sci 20:307–317. https://doi.org/10.1111/1744-7917.12002
Chiremba C, Taylor JRN, Duodu KG (2009) Phenolic content, antioxidant activity, and consumer acceptability of sorghum cookies. Cereal Chem 86:590–594. https://doi.org/10.1094/CCHEM-86-5-0590
Chong J, Poutaraud A, Hugueney P (2009) Metabolism and roles of stilbenes in plants. Plant Sci 177:143–155
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352. https://doi.org/10.3390/molecules15107313
De Mey Y, Demont M, Diagne M (2012) Estimating bird damage to rice in Africa: evidence from the senegal river valley. J Agric Econ 63:175–200. https://doi.org/10.1111/j.1477-9552.2011.00323.x
de Morais CL, Pinheiro SS, Martino HSD, Pinheiro-Sant’Ana HM (2017) Sorghum (Sorghum bicolor L.): nutrients, bioactive compounds, and potential impact on human health. Crit Rev Food Sci Nutr 57:372–390
Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF (2011) Salicylic acid biosynthesis and metabolism. Arab B 9:e0156. https://doi.org/10.1199/tab.0156
Deng Y, Lu S (2017) Biosynthesis and regulation of phenylpropanoids in plants. CRC Crit Rev Plant Sci 36:1–34. https://doi.org/10.1080/07352689.2017.1402852
Devi PS, Kumar MS, Das SM (2011) Evaluation of antiproliferative activity of red Sorghum Bran Anthocyanin on a human breast cancer cell line (MCF-7). Int J Breast Cancer 2011:1–6. https://doi.org/10.4061/2011/891481
Dicko MH, Gruppen H, Barro C et al (2005) Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J Chem Ecol 31:2671–2688. https://doi.org/10.1007/s10886-005-7619-5
Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins—A final frontier in flavonoid research? New Phytol 165:9–28. https://doi.org/10.1111/j.1469-8137.2004.01217.x
Dreyer DL, Jones KC (1981) Feeding deterrency of flavonoids and related phenolics towards Schizaphis graminum and Myzus persicae: aphid feeding deterrents in wheat. Phytochemistry 20:2489–2493. https://doi.org/10.1016/0031-9422(81)83078-6
Dykes L (2019) Sorghum phytochemicals and their potential impact on human health. Methods Mol Biol 1931:121–140. https://doi.org/10.1007/978-1-4939-9039-9_9
Dykes L, Rooney LW (2006) Sorghum and millet phenols and antioxidants. J Cereal Sci 44:236–251. https://doi.org/10.1016/j.jcs.2006.06.007
Dykes L, Peterson GC, Rooney WL, Rooney LW (2011) Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem 128:173–179. https://doi.org/10.1016/j.foodchem.2011.03.020
Dykes L, Rooney WL, Rooney LW (2013) Evaluation of phenolics and antioxidant activity of black sorghum hybrids. J Cereal Sci 58:278–283. https://doi.org/10.1016/j.jcs.2013.06.006
Eastin JD, Lee K-W (2020) Sorghum bicolor. CRC handbook of flowering. Springer, CRC Press, pp 367–375
Eigenbrode SD, Espelie KE (1995) Effects of plant epicuticular lipids on insect herbivores. Annu Rev Entomol 40:171–194. https://doi.org/10.1146/annurev.en.40.010195.001131
Esele JP (1993) The association of genes controlling caryopsis traits with grain mold resistance in Sorghum. Phytopathology 83:490. https://doi.org/10.1094/phyto-83-490
Floegel A, Kim DO, Chung SJ et al (2010) Development and validation of an algorithm to establish a total antioxidant capacity database of the US diet. Int J Food Sci Nutr 61:600–623. https://doi.org/10.3109/09637481003670816
Garzón AG, Torres RL, Drago SR (2019) Changes in phenolics, γ-aminobutyric acid content and antioxidant, antihypertensive and hypoglycaemic properties during ale white sorghum (Sorghum bicolor (L.) Moench) brewing process. Int J Food Sci Technol 54:1901–1908. https://doi.org/10.1111/ijfs.14102
Genzeng R, Jianghui C, Xiaodong X et al (2019) Flavonoid biosynthesis pathway participating in salt resistance in a landrace sweet Sorghum revealed by RNA-sequencing comparison with grain Sorghum. J Agric Sci 11:63. https://doi.org/10.5539/jas.v11n6p63
Girard AL, Awika JM (2018) Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J Cereal Sci 84:112–124. https://doi.org/10.1016/j.jcs.2018.10.009
Girard AL, Teferra T, Awika JM (2019) Effects of condensed vs hydrolysable tannins on gluten film strength and stability. Food Hydrocoll 89:36–43. https://doi.org/10.1016/j.foodhyd.2018.10.018
González-Montilla FM, Chávez-Santoscoy RA, Gutiérrez-Uribe JA, Serna-Saldivar SO (2012) Isolation and identification of phase II enzyme inductors obtained from black Shawaya sorghum [Sorghum bicolor (L.) Moench] bran. J Cereal Sci 55:126–131
Greenberg AS, Obin MS (2006) Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 83:461S-465S. https://doi.org/10.1093/ajcn/83.2.461s
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445. https://doi.org/10.1146/annurev-immunol-031210-101322
Hoi JT, Weller CL, Schlegel VL et al (2009) Sorghum distillers dried grain lipid extract increases cholesterol excretion and decreases plasma and liver cholesterol concentration in hamsters. J Funct Foods 1:381–386. https://doi.org/10.1016/j.jff.2009.09.005
Hole AS, Grimmer S, Jensen MR, Sahlstrøm S (2012) Synergistic and suppressive effects of dietary phenolic acids and other phytochemicals from cereal extracts on nuclear factor kappa B activity. Food Chem 133:969–977. https://doi.org/10.1016/j.foodchem.2012.02.017
Ibraheem F, Gaffoor I, Chopra S (2010) Flavonoid phytoalexin-dependent resistance to anthracnose leaf blight requires a functional yellow seed1 in Sorghum bicolor. Genetics 184:915–926. https://doi.org/10.1534/genetics.109.111831
Jideani AI, Silungwe H, Takalani T et al (2014) Antioxidant-rich natural grain products and human health. In: Oguntibeju O (ed) Antioxidant-antidiabetic agents hum heal. InTech Publ Rijeka, Croat, pp 167–187
Kam J, Puranik S, Yadav R et al (2016) Dietary interventions for type 2 diabetes: How millet comes to help. Front Plant Sci 7:1454. https://doi.org/10.3389/fpls.2016.01454
Kamath VG, Chandrashekar A, Rajini PS (2004) Antiradical properties of sorghum (Sorghum bicolor L. Moench) flour extracts. J Cereal Sci 40:283–288. https://doi.org/10.1016/j.jcs.2004.08.004
Kariyat RR, Smith JD, Stephenson AG et al (2017) Non-glandular trichomes of solanum carolinense deter feeding by manduca sexta caterpillars and cause damage to the gut peritrophic matrix. Proc R Soc B Biol Sci 284:20162323. https://doi.org/10.1098/rspb.2016.2323
Kayodé APP, Nout MJR, Linnemann AR et al (2011) Uncommonly high levels of 3-deoxyanthocyanidins and antioxidant capacity in the leaf sheaths of dye sorghum. J Agric Food Chem 59:1178–1184
Kil HY, Seong ES, Ghimire BK et al (2009) Antioxidant and antimicrobial activities of crude sorghum extract. Food Chem 115:1234–1239. https://doi.org/10.1016/j.foodchem.2009.01.032
Kim Y, Park N (2012) Development and application of STEAM teaching model based on the Rube Goldberg’s invention. In: Yeo S-S, Pan Y, Lee YS, Chang HB (eds) Lecture notes in electrical engineering. Springer, Dordrecht, pp 693–698
Klopfenstein T, Owen FG (1981) Value and potential use of crop residues and by-products in dairy rations. J Dairy Sci 64:1250–1268. https://doi.org/10.3168/jds.S0022-0302(81)82699-9
Kruger J, Taylor JRN, Du X et al (2013) Effect of phytate reduction of sorghum, through genetic modification, on iron and zinc availability as assessed by an in vitro dialysability bioaccessibility assay, Caco-2 cell uptake assay, and suckling rat pup absorption model. Food Chem 141:1019–1025. https://doi.org/10.1016/j.foodchem.2013.01.105
Kumar N, Goel N (2019) Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep 24:370
Kumari P, Pahuja SK, Arya S, Patil JV (2016) Sorghum. In: Singh M, Kumar S (eds) Broadening the genetic base of grain cereals. Springer, New Delhi, pp 163–203
Kumari P, Pahuja SK, Sheoran RS et al (2017) Effect of varying levels of salinity on growth, yield and quality of forage sorghum genotypes. Forage Res 43:64–66
Laxmi V, Pahuja SK, Kumari P (2019) Identification of new sources for good quality high biomass yield and other promising traits in mini core collection of forage Sorghum. Indian J Plant Genet Resour 32:150–157
Lee H, Peirsman Y, Chang A, et al (2011) Standford’s multi-pass sieve coreference resolution system at the CoNLL-2011. In: Fifteenth conference on computational natural language learning. Association for Computational Linguistics, pp 28–34
Lee SM, Pan BS (2003) Inhibitory effect of tannin in dietary sorghum distillery residue and preliminary treatment with polyethylene glycol on in vitro digestibility of grey mullet (mugil cephalus). J Food Biochem 27:485–500. https://doi.org/10.1111/j.1745-4514.2003.tb00596.x
Lewis JB, Taddeo SS, McDonough CM et al (2008) Sorghum bran varieties differentially influence endogenous antioxidant enzymes to protect against oxidative stress during colon carcinogenesis. Faseb J 22:887.7
Links MR, Taylor J, Kruger MC, Taylor JRN (2015) Sorghum condensed tannins encapsulated in kafirin microparticles as a nutraceutical for inhibition of amylases during digestion to attenuate hyperglycaemia. J Funct Foods 12:55–63. https://doi.org/10.1016/j.jff.2014.11.003
Liu G, Godwin ID (2012) Highly efficient sorghum transformation. Plant Cell Rep 31:999–1007. https://doi.org/10.1007/s00299-011-1218-4
Loeh SCC, De Verdier K, Nicholson RL (1999) Accumulation of 3-deoxyanthocyanidin phytoalexins and resistance to Colletotrichum sublineolum in sorghum. Physiol Mol Plant Pathol 55:263–273. https://doi.org/10.1006/pmpp.1999.0231
Luckemeyer TJ, Miller RK, Kerth CR, Adhikari K (2016) Beef flavor attributes and consumer perception II. Meat Sci 112:114
Makoi JHJR, Belane AK, Chimphango SBM, Dakora FD (2010) Seed flavonoids and anthocyanins as markers of enhanced plant defence in nodulated cowpea (Vigna unguiculata L. Walp.). F Crop Res 118:21–27. https://doi.org/10.1016/j.fcr.2010.03.012
Mehmood S, Bashir A, Ahmad A et al (2008) Molecular characterization of regional sorghum bicolor varieties from Pakistan. Pakistan J Bot 40:2015–2021
Melake-Berhan A, Butler LG, Ejeta G, Menkir A (1996) Grain mold resistance and polyphenol accumulation in Sorghum. J Agric Food Chem 44:2428–2434. https://doi.org/10.1021/jf950580x
Meng C, Zhang S, Deng Y-S et al (2015) Overexpression of a tomato flavanone 3-hydroxylase-like protein gene improves chilling tolerance in tobacco. Plant Physiol Biochem 96:388–400
Mkandawire NL, Kaufman RC, Bean SR et al (2013) Effects of sorghum (Sorghum bicolor (L.) Moench) tannins on α-amylase activity and in vitro digestibility of starch in raw and processed flours. J Agric Food Chem 61:4448–4454. https://doi.org/10.1021/jf400464j
Mokrane H, Amoura H, Belhaneche-Bensemra N et al (2010) Assessment of Algerian sorghum protein quality [Sorghum bicolor (L.) Moench] using amino acid analysis and in vitro pepsin digestibility. Food Chem 121:719–723. https://doi.org/10.1016/j.foodchem.2010.01.020
Moraes ÉA, Natal DIG, Queiroz VAV et al (2012) Sorghum genotype may reduce low-grade inflammatory response and oxidative stress and maintains jejunum morphology of rats fed a hyperlipidic diet. Food Res Int 49:553–559. https://doi.org/10.1016/j.foodres.2012.07.029
Mote UN, Shahane AK (1993) Studies on varietal reaction of sorghum to Delphacid, aphid and leaf sugary exudation. Indian J Entomol 55:360
Muriu JI, Njoka-Njiru EN, Tuitoek JK, Nanua JN (2002) Evaluation of sorghum (Sorghum bicolor) as replacent for maize in the diet of growing rabbits (Oryctolagus cuniculus). Asian-Australas J Anim Sci 15:565–569. https://doi.org/10.5713/ajas.2002.565
Musa EM, Elsheikh EAE, Ahmed IAM, Babiker EE (2011) Effect of intercropping with cowpea (Vigna unuiculata L.), Bradyrhizobium inoculation and fertilization on physical and biochemical quality of sorghum (Sorghum bicolor L.) seeds. Electron J Environ Agric Food Chem 10:3064–3075
N’Dri D, Mazzeo T, Zaupa M et al (2013) Effect of cooking on the total antioxidant capacity and phenolic profile of some whole-meal African cereals. J Sci Food Agric 93:29–36. https://doi.org/10.1002/jsfa.5837
Nida H, Girma G, Mekonen M et al (2021) Genome-wide association analysis reveals seed protein loci as determinants of variations in grain mold resistance in sorghum. Theor Appl Genet 134:1167–1184. https://doi.org/10.1007/s00122-020-03762-2
Ofosu FK, Elahi F, Daliri EBM et al (2021) UHPLC-ESI-QTOF-MS/MS characterization, antioxidant and antidiabetic properties of sorghum grains. Food Chem 337:127788. https://doi.org/10.1016/j.foodchem.2020.127788
Ogurtsova K, da Rocha Fernandes JD, Huang Y et al (2017) IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50. https://doi.org/10.1016/j.diabres.2017.03.024
Petti C, Hirano K, Stork J, DeBolt S (2015) Mapping of a cellulose-deficient mutant named dwarf1-1 in Sorghum bicolor to the green revolution gene gibberellin20-oxidase reveals a positive regulatory association between gibberellin and cellulose biosynthesis. Plant Physiol 169:705–716. https://doi.org/10.1104/pp.15.00928
Pomerenke JL, Souza LWO, Shurson GC (2010) Concentrations of β-glucans and mannan oligosaccharides in corn dried distillers grains with soluble (DDGS) and its relationship to fiber components. J Anim Sci 93:206
Reinisalo M, Kårlund A, Koskela A et al (2015) Polyphenol stilbenes: molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid Med Cell Longev. https://doi.org/10.1155/2015/340520
Salazar-López NJ, González-Aguilar G, Rouzaud-Sández O, Robles-Sánchez M (2018) Technologies applied to sorghum (Sorghum bicolor l. moench): changes in phenolic compounds and antioxidant capacity. Food Sci Technol 38:369–382. https://doi.org/10.1590/fst.16017
Sánchez-Zapata E, Viuda-Martos M, Fernández-LÓpez J, Pérez-Alvarez JA (2015) Resistant starch as functional ingredient. Polysaccharides Bioactivity Biotechnol 43:1911–1931. https://doi.org/10.1007/978-3-319-16298-0_34
Saura-Calixto F, Pérez-Jiménez J, Touriño S et al (2010) Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol Nutr Food Res 54:939–946. https://doi.org/10.1002/mnfr.200900276
Seneviratne G, Jayasinghearachchi HS (2003) Phenolic acids: Possible agents of modifying N 2-fixing symbiosis through rhizobial alteration? Plant Soil 252:385–395
Sharma HC (1993) Host-plant resistance to insects in sorghum and its role in integrated pest management. Crop Prot 12:11–34. https://doi.org/10.1016/0261-2194(93)90015-B
Sharma S, Verslues PE (2010) Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ 33:1838–1851. https://doi.org/10.1111/j.1365-3040.2010.02188.x
Sharma A, Shahzad B, Rehman A et al (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24:2452
Singh V, Moreau RA, Hicks KB (2003) Yield and phytosterol composition of oil extracted from grain sorghum and its wet-milled fractions. Cereal Chem 80:126–129. https://doi.org/10.1094/CCHEM.2003.80.2.126
Stefoska-Needham A, Beck EJ, Johnson SK et al (2017) A diet enriched with red sorghum flaked biscuits, compared to a diet containing white wheat flaked biscuits, does not enhance the effectiveness of an energy-restricted meal plan in overweight and mildly obese adults. J Am Coll Nutr 36:184–192. https://doi.org/10.1080/07315724.2016.1237314
Takabe T, Rai V, Hibino T (2006) Metabolic engineering of glycine betaine. In: Rai AK, Takabe T (eds) Abiotic stress tolerance in plants. Springer, Dordrecht, pp 137–151
Taleon V, Dykes L, Rooney WL, Rooney LW (2012) Effect of genotype and environment on flavonoid concentration and profile of black sorghum grains. J Cereal Sci 56:470–475. https://doi.org/10.1016/j.jcs.2012.05.001
Tipton KW, Floyd EH, Marshall JG, McDevitt JB (1970) Resistance of certain grain sorghum hybrids to bird damage in Louisiana 1. Agron J 62:211–213. https://doi.org/10.2134/agronj1970.00021962006200020010x
Tugizimana F, Djami-Tchatchou AT, Steenkamp PA et al (2019) Metabolomic analysis of defense-related reprogramming in Sorghum bicolor in response to Colletotrichum sublineolum infection reveals a functional metabolic web of phenylpropanoid and flavonoid pathways. Front Plant Sci 9:1840
Vyas P, Singh D, Singh N et al (2018) Nutrigenomics: advances, opportunities and challenges in understanding the nutrient-gene interactions. Curr Nutr Food Sci 14:104–115. https://doi.org/10.2174/1573401313666170614094410
Walling LL (2008) Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol 146:859–866. https://doi.org/10.1104/pp.107.113142
Wani ZA, Ashraf N, Mohiuddin T, Riyaz-Ul-Hassan S (2015) Plant-endophyte symbiosis, an ecological perspective. Appl Microbiol Biotechnol 99:2955–2965
Wedick NM, Ma Y, Olendzki BC et al (2015) Access to healthy food stores modifies effect of a dietary intervention. Am J Prev Med 48:309–317. https://doi.org/10.1016/j.amepre.2014.08.020
Weir TL, Park SW, Vivanco JM (2004) Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol 7:472–479. https://doi.org/10.1016/j.pbi.2004.05.007
Williams RJ, Rao KN (1981) A review of sorghum grain moulds. Trop Pest Manag 27:200–211. https://doi.org/10.1080/09670878109413652
Xie Q, Xu Z (2019) Sustainable agriculture: from sweet sorghum planting and ensiling to ruminant feeding. Mol Plant 12:603–606. https://doi.org/10.1016/j.molp.2019.04.001
Xiong Y, Zhang P, Warner RD, Fang Z (2019) Sorghum grain: from genotype, nutrition, and phenolic profile to its health benefits and food applications. Compr Rev Food Sci Food Saf 18:2025–2046. https://doi.org/10.1111/1541-4337.12506
Xiong Y, Teixeira TV, Zhang P, Warner RD, Shen S, Fang Z (2021) Cellular antioxidant activities of phenolic extracts from five sorghum grain genotypes. Food Biosci 41:101068
Yang L, Browning JD, Awika JM (2009) Sorghum 3-deoxyanthocyanins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. J Agric Food Chem 57:1797–1804. https://doi.org/10.1021/jf8035066
Young WR, Teetes GL (1977) Sorghum entomology. Annu Rev Entomol 22:193–218
Acknowledgements
The Authors would like to thank Dr. Nisha Thakur, Assistant Professor (English), Himachal Pradesh University, Shimla, India, and Falcon Scientific Editing (https://falconediting.com) for proofreading the English language in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human participants and/or animals
Research did not involve human and/or animal subjects.
Informed consent
All authors have read and approved the submission of this work.
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s00425-021-03748-4
About this article
Cite this article
Kumari, P., Kumar, V., Kumar, R. et al. RETRACTED ARTICLE: Sorghum polyphenols: plant stress, human health benefits, and industrial applications. Planta 254, 47 (2021). https://doi.org/10.1007/s00425-021-03697-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03697-y