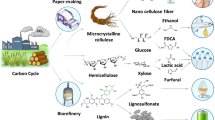
Abstract
Lignin fractionation and depolymerization generates heterogeneous streams of aromatic compounds and conversion of aromatic compounds into valuable products, but it is not efficient. Many microbes in nature have evolved metabolic pathways to convert complex lignin polymers into aromatic compounds and transform these aromatic compounds into central intermediates for bioproduct synthesis. The objective of this paper is to review the recent process development of lignin bioconversion into aromatic compounds and bioproducts. Lignin structural and molecular changes during fractionation and depolymerization are presented. Subsequent lignin conversion into aromatic compounds by upper pathways and further converted into central metabolites and bioproducts via lower pathways are emphasized. In particular, enzymes and mediator systems to enhance lignin conversion and key intermediates in lignin catabolic pathways are discussed. Strategies to enhance bioproduct formation through lignin valorization are summarized.


Similar content being viewed by others
References
Linger JG, Vardon DR, Guarnieri MT, Karp EM, Hunsinger GB, Franden MA, et al. Lignin valorization through integrated biological funneling and chemical catalysis. Proc Natl Acad Sci. 2014;111(33):12013–8.
Brebu M, Cazacu G, Chirila O. Pyrolysis of lignin-a potential method for obtaining chemicals and/or fuels. Cellul Chem Technol. 2011;45(1):43.
De la Torre MJ, Moral A, Hernandez MD, Cabeza E, Tijero A. Organosolv lignin for biofuel. Ind Crops Prod. 2013;45:58–63.
Sannigrahi P, Pu Y, Ragauskas A. Cellulosic biorefineries-unleashing lignin opportunities. Curr Opin Environ Sustain. 2010;2(5–6):383–93.
Becker J, Wittmann C. A field of dreams: lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol Adv. 2019;37(6):107360. https://doi.org/10.1016/j.biotechadv.2019.02.016.
Sun X, Li M, Chen Y. Biochar facilitated bioprocessing and biorefinery for productions of biofuel and chemicals: a review. Bioresour Technol. 2019. https://doi.org/10.1016/j.biortech.2019.122252.
Li M, Marek SM, Peng J, Liu Z, Wilkins MR. Effect of moisture content and inoculum size on cell wall composition and ethanol yield from switchgrass after solid-state Pleurotus ostreatus treatment. Trans ASABE. 2018;61(6):1997–2006. https://doi.org/10.13031/trans.12981.
Ding C, Li M, Hu Y. High-activity production of xylanase by Pichia stipitis: purification, characterization, kinetic evaluation and xylooligosaccharides production. Int J Biol Macromol. 2018;117:72–7.
Frederick N, Li M, Carrier DJ, Buser MD, Wilkins MR. Switchgrass storage effects on the recovery of carbohydrates after liquid hot water pretreatment and enzymatic hydrolysis. AIMS Bioeng. 2016;3(3):389–99. https://doi.org/10.3934/bioeng.2016.3.389.
Chen Z, Wan C. Co-fermentation of lignocellulose-based glucose and inhibitory compounds for lipid synthesis by Rhodococcus jostii RHA1. Process Biochem. 2017;57:159–66.
Liu K, Atiyeh HK, Pardo-Planas O, Ezeji TC, Ujor V, Overton JC, et al. Butanol production from hydrothermolysis-pretreated switchgrass: quantification of inhibitors and detoxification of hydrolyzate. Bioresour Technol. 2015;189:292–301.
Ding C, Wang X, Li M. Evaluation of six white-rot fungal pretreatments on corn stover for the production of cellulolytic and ligninolytic enzymes, reducing sugars, and ethanol. Appl Microbiol Biotechnol. 2019. https://doi.org/10.1007/s00253-019-09884-y.
Li M, Eskridge K, Liu E, Wilkins M. Enhancement of polyhydroxybutyrate (PHB) production by 10-fold from alkaline pretreatment liquor with an oxidative enzyme-mediator-surfactant system under Plackett–Burman and central composite designs. Bioresour Technol. 2019;281:99–106. https://doi.org/10.1016/j.biortech.2019.02.045.
Li M, Eskridge KM, Wilkins MR. Optimization of polyhydroxybutyrate production by experimental design of combined ternary mixture (glucose, xylose and arabinose) and process variables (sugar concentration, molar C:N ratio). Bioprocess Biosyst Eng. 2019. https://doi.org/10.1007/s00449-019-02146-1.
Ramachandriya KD, Wilkins M, Atiyeh HK, Dunford NT, Hiziroglu S. Effect of high dry solids loading on enzymatic hydrolysis of acid bisulfite pretreated Eastern redcedar. Bioresour Technol. 2013;147:168–76.
Faga BA, Wilkins MR, Banat IM. Ethanol production through simultaneous saccharification and fermentation of switchgrass using Saccharomyces cerevisiae D5A and thermotolerant Kluyveromyces marxianus IMB strains. Bioresour Technol. 2010;101(7):2273–9. https://doi.org/10.1016/j.biortech.2009.11.001.
Zhang X, Tang H, Chen G, Qiao L, Li J, Liu B, et al. Growth performance and nutritional profile of mealworms reared on corn stover, soybean meal, and distillers’ grains. Eur Food Res Technol. 2019;245:2631–40.
Li M. White rot fungi Pleurotus ostreatus pretreatment on switchgrass to enhance enzymatic hydrolysis and ethanol production. Stillwater: Oklahoma State University; 2015.
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, et al. Lignin valorization: improving lignin processing in the biorefinery. Science (Wash D C). 2014;344(6185):1246843.
Li M, Wilkins MR. Recent advances in polyhydroxyalkanoate production: feedstocks, strains and process developments. Int J Biol Macromol. 2020;156:691–703.
Li M. Adding value to lignocellulosic biorefinery: efficient process development of lignocellulosic biomass conversion into polyhydroxybutyrate. [Dissertation]. Lincoln: University of Nebraska-Lincoln; 2019.
Sun Z, Fridrich B, De Santi A, Elangovan S, Barta K. Bright side of lignin depolymerization: toward new platform chemicals. Chem Rev. 2018;118(2):614–78.
Schutyser W, Renders T, Van den Bosch S, Koelewijn S-F, Beckham GT, Sels BF. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. ChSRv. 2018;47(3):852–908.
Kim J-Y, Lee HW, Lee SM, Jae J, Park Y-K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour Technol. 2019;279:373–84. https://doi.org/10.1016/j.biortech.2019.01.055.
Wang H, Pu Y, Ragauskas A, Yang B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour Technol. 2019;271:449–61. https://doi.org/10.1016/j.biortech.2018.09.072.
Abdelaziz OY, Brink DP, Prothmann J, Ravi K, Sun M, Garcia-Hidalgo J, et al. Biological valorization of low molecular weight lignin. Biotechnol Adv. 2016;34(8):1318–46.
Ahmad E, Pant KK. Chapter 14—lignin conversion: A key to the concept of lignocellulosic biomass-based integrated biorefinery. In: Bhaskar T, Pandey A, Mohan SV, Lee D-J, Khanal SK, editors. Waste biorefinery. Amsterdam: Elsevier; 2018. p. 409–444.
Vanholme R, Morreel K, Darrah C, Oyarce P, Grabber JH, Ralph J, et al. Metabolic engineering of novel lignin in biomass crops. New Phytol. 2012;196(4):978–1000.
Tolbert A, Akinosho H, Khunsupat R, Naskar AK, Ragauskas AJ. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod Biorefin. 2014;8(6):836–56.
Ralph J. Hydroxycinnamates in lignification. Phytochem Rev. 2010;9(1):65–83.
Del Rio JC, Rencoret J, Prinsen P, Martinez AT, Ralph J, Gutierrez A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J Agric Food Chem. 2012;60(23):5922–35.
Lan W, Morreel K, Lu F, Rencoret J, del Río JC, Voorend W, et al. Maize tricin-oligolignol metabolites and their implications for monocot lignification. Plant Physiol. 2016;2016:02012.
Morreel K, Ralph J, Kim H, Lu F, Goeminne G, Ralph S, et al. Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiol. 2004;136(3):3537–49.
Karp EM, Nimlos CT, Deutch S, Salvachua D, Cywar RM, Beckham GT. Quantification of acidic compounds in complex biomass-derived streams. Green Chem. 2016;18(17):4750–60.
Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, et al. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev. 2004;3(1–2):29–60.
Ralph J, Akiyama T, Kim H, Lu F, Schatz PF, Marita JM, et al. Effects of coumarate 3-hydroxylase down-regulation on lignin structure. J Biol Chem. 2006;281(13):8843–53.
Bugg TD, Ahmad M, Hardiman EM, Rahmanpour R. Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep. 2011;28(12):1883–966.
Liu Z-H, Olson ML, Shinde S, Wang X, Hao N, Yoo CG, et al. Synergistic maximization of the carbohydrate output and lignin processability by combinatorial pretreatment. Green Chem. 2017;19(20):4939–55.
Holladay JE, White JF, Bozell JJ, Johnson D. Top value-added chemicals from biomass-Volume II—Results of screening for potential candidates from biorefinery lignin. Richland: Pacific Northwest National Lab (PNNL); 2007.
Eggeman T, Elander RT. Process and economic analysis of pretreatment technologies. Bioresour Technol. 2005;96(18):2019–25.
Ralph J, Lapierre C, Boerjan W. Lignin structure and its engineering. Curr Opin Biotechnol. 2019;56:240–9. https://doi.org/10.1016/j.copbio.2019.02.019.
Salvachua D, Karp EM, Nimlos CT, Vardon DR, Beckham GT. Towards lignin consolidated bioprocessing: simultaneous lignin depolymerization and product generation by bacteria. Green Chem. 2015;17(11):4951–67.
Rinaldi R, Jastrzebski R, Clough MT, Ralph J, Kennema M, Bruijnincx PC, et al. Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew Chem Int Ed. 2016;55(29):8164–215.
Azadi P, Inderwildi OR, Farnood R, King DA. Liquid fuels, hydrogen and chemicals from lignin: a critical review. Renew Sustain Energy Rev. 2013;21:506–23.
Lange JP. Renewable feedstocks: the problem of catalyst deactivation and its mitigation. Angew Chem Int Ed. 2015;54(45):13186–97.
Laurichesse S, Averous L. Chemical modification of lignins: towards biobased polymers. Prog Polym Sci. 2014;39(7):1266–90.
Wang Y, Wang Q, He J, Zhang Y. Highly effective C–C bond cleavage of lignin model compounds. Green Chem. 2017;19(13):3135–41.
Macfarlane A, Mai M, Kadla J. Bio-based chemicals from biorefining: lignin conversion and utilisation. In: Waldron KW, editor. Advances in biorefineries. 1st ed. Cambridge: Elsevier; 2014. p. 659–692.
Kim S, Holtzapple MT. Lime pretreatment and enzymatic hydrolysis of corn stover. Bioresour Technol. 2005;96(18):1994–2006. https://doi.org/10.1016/j.biortech.2005.01.014.
Karp EM, Donohoe BS, O’Brien MH, Ciesielski PN, Mittal A, Biddy MJ, et al. Alkaline pretreatment of corn stover: bench-scale fractionation and stream characterization. ACS Sustain Chem Eng. 2014;2(6):1481–91.
Karp EM, Resch MG, Donohoe BS, Ciesielski PN, O’Brien MH, Nill JE, et al. Alkaline pretreatment of switchgrass. ACS Sustain Chem Eng. 2015;3(7):1479–91.
Chen Y, Stevens MA, Zhu Y, Holmes J, Xu H. Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol Biofuels. 2013;6(1):8.
Mittal A, Katahira R, Donohoe BS, Pattathil S, Kandemkavil S, Reed ML, et al. Ammonia pretreatment of corn stover enables facile lignin extraction. ACS Sustain Chem Eng. 2017;5(3):2544–61.
Da Costa SL, Jin M, Chundawat SP, Bokade V, Tang X, Azarpira A, et al. Next-generation ammonia pretreatment enhances cellulosic biofuel production. Energy Environ Sci. 2016;9(4):1215–23.
Chen X, Tao L, Shekiro J, Mohaghaghi A, Decker S, Wang W, et al. Improved ethanol yield and reduced Minimum Ethanol Selling Price (MESP) by modifying low severity dilute acid pretreatment with deacetylation and mechanical refining: (1) Experimental. Biotechnol Biofuels. 2012;5(1):60.
Xu J, Cheng JJ. Pretreatment of switchgrass for sugar production with the combination of sodium hydroxide and lime. Bioresour Technol. 2011;102(4):3861–8. https://doi.org/10.1016/j.biortech.2010.12.038.
Wyman CE. Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. West Sussex: Wiley; 2013.
Da Costa SL, Foston M, Bokade V, Azarpira A, Lu F, Ragauskas AJ, et al. Isolation and characterization of new lignin streams derived from extractive-ammonia (EA) pretreatment. Green Chem. 2016;18(15):4205–15.
Si M, Yan X, Liu M, Shi M, Wang Z, Wang S, et al. In situ lignin bioconversion promotes complete carbohydrate conversion of rice straw by Cupriavidus basilensis B-8. ACS Sustain Chem Eng. 2018;6(6):7969–78.
Vvu S, Argyropoulos D. An improved method for isolating lignin in high yield and purity. J Pulp Pap Sci. 2003;29:235–40.
Salvachua D, Katahira R, Cleveland NS, Khanna P, Resch MG, Black BA, et al. Lignin depolymerization by fungal secretomes and a microbial sink. Green Chem. 2016;18(22):6046–62.
Kaldstrom M, Meine N, Fares C, Rinaldi R, Schuth F. Fractionation of water-soluble lignocellulose into C 5/C 6 sugars and sulfur-free lignins. Green Chem. 2014;16(5):2454–62.
Kaldstrom M, Meine N, Fares C, Schuth F, Rinaldi R. Deciphering water-soluble lignocellulose obtained by mechanocatalysis: new insights into the chemical processes leading to deep depolymerization. Green Chem. 2014;16(7):3528–38.
Calvaruso G, Clough MT, Rechulski MDK, Rinaldi R. On the meaning and origins of lignin recalcitrance: a critical analysis of the catalytic upgrading of lignins obtained from mechanocatalytic biorefining and organosolv pulping. ChemCatChem. 2017;9(14):2691–700.
Singh S, Cheng G, Sathitsuksanoh N, Wu D, Varanasi P, George A, et al. Comparison of different biomass pretreatment techniques and their impact on chemistry and structure. Front Energy Res. 2015;2:62.
Martinez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, et al. Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol. 2005;8:195–204.
Gomez-Toribio V, Garcia-Martin AB, Martínez MJ, MartInez AT, Guillen F. Induction of extracellular hydroxyl radical production by white-rot fungi through quinone redox cycling. Appl Environ Microbiol. 2009;75(12):3944–53.
Fuchs G, Boll M, Heider J. Microbial degradation of aromatic compounds—from one strategy to four. Nat Rev Microbiol. 2011;9(11):803.
Seaton SC, Neidle EL. Using aerobic pathways for aromatic compound degradation to engineer lignin metabolism. In: Beckham GT, editor. Lignin valorization. 1st ed. London: The Royal Society of Chemistry; 2018. p. 252–289.
Boll M, Loffler C, Morris BE, Kung JW. Anaerobic degradation of homocyclic aromatic compounds via arylcarboxyl-coenzyme A esters: organisms, strategies and key enzymes. Environ Microbiol. 2014;16(3):612–27.
Kosa M, Ragauskas AJ. Bioconversion of lignin model compounds with oleaginous Rhodococci. Appl Microbiol Biotechnol. 2012;93(2):891–900.
He Y, Li X, Ben H, Xue X, Yang B. Lipid production from dilute alkali corn stover lignin by Rhodococcus strains. ACS Sustain Chem Eng. 2017;5(3):2302–11.
Majumdar S, Lukk T, Solbiati JO, Bauer S, Nair SK, Cronan JE, et al. Roles of small laccases from Streptomyces in lignin degradation. Biochemistry. 2014;53(24):4047–58.
Taylor C, Hardiman E, Ahmad M, Sainsbury P, Norris P, Bugg T. Isolation of bacterial strains able to metabolize lignin from screening of environmental samples. J Appl Microbiol. 2012;113(3):521–30.
Bandounas L, Wierckx NJ, de Winde JH, Ruijssenaars HJ. Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnol. 2011;11(1):94.
Masai E, Katayama Y, Fukuda M. Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem. 2007;71(1):1–15.
Beckham GT, Johnson CW, Karp EM, Salvachua D, Vardon DR. Opportunities and challenges in biological lignin valorization. Curr Opin Biotechnol. 2016;42:40–53. https://doi.org/10.1016/j.copbio.2016.02.030.
Chen Z, Wan C. Biological valorization strategies for converting lignin into fuels and chemicals. Renew Sustain Energy Rev. 2017;73:610–21.
Paliwal R, Rawat AP, Rawat M, Rai JPN. Bioligninolysis: recent updates for biotechnological solution. Appl Biochem Biotechnol. 2012;167(7):1865–89. https://doi.org/10.1007/s12010-012-9735-3.
Liu E, Li M, Abdella A, Wilkins MR. Development of a cost-effective medium for submerged production of fungal aryl alcohol oxidase using a genetically modified Aspergillus nidulans strain. Bioresour Technol. 2020;305:123038. https://doi.org/10.1016/j.biortech.2020.123038.
Chio C, Sain M, Qin W. Lignin utilization: a review of lignin depolymerization from various aspects. Renew Sustain Energy Rev. 2019;107:232–49. https://doi.org/10.1016/j.rser.2019.03.008.
Bourbonnais R, Paice MG. Oxidation of non-phenolic substrates. FEBS Lett. 1990;267(1):99–102.
Camarero S, Garcıa O, Vidal T, Colom J, del Rıo JC, Gutiérrez A, et al. Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microb Technol. 2004;35(2–3):113–20.
Shleev S, Persson P, Shumakovich G, Mazhugo Y, Yaropolov A, Ruzgas T, et al. Interaction of fungal laccases and laccase-mediator systems with lignin. Enzyme Microb Technol. 2006;39(4):841–7.
Eggert C, Temp U, Eriksson K-E. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol. 1996;62(4):1151–8.
Martínkova L, Kotik M, Markova E, Homolka L. Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: a review. Chemosphere. 2016;149:373–82.
Tagger S, Perissol C, Gil G, Vogt G, Le Petit J. Phenoloxidases of the white-rot fungus Marasmius quercophilus isolated from an evergreen oak litter (Quercus ilex L.). Enzyme Microb Technol. 1998;23(6):372–9.
Wesenberg D, Kyriakides I, Agathos SN. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv. 2003;22(1):161–87. https://doi.org/10.1016/j.biotechadv.2003.08.011.
Chandra R, Chowdhary P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ Sci Processes Impacts. 2015;17(2):326–42.
Liu D, Yan X, Zhuo S, Si M, Liu M, Wang S, et al. Pandoraea sp B-6 assists the deep eutectic solvent pretreatment of rice straw via promoting lignin depolymerization. Bioresour Technol. 2018;257:62–8. https://doi.org/10.1016/j.biortech.2018.02.029.
Christopher LP, Yao B, Ji Y. Lignin biodegradation with laccase-mediator systems. Front Energy Res. 2014. https://doi.org/10.3389/fenrg.2014.00012.
Raj K, Krishnan C. High sugar yields from sugarcane (Saccharum officinarum) bagasse using low-temperature aqueous ammonia pretreatment and laccase-mediator assisted enzymatic hydrolysis. Ind Crops Prod. 2018;111:673–83. https://doi.org/10.1016/j.indcrop.2017.11.047.
Brenelli L, Squina FM, Felby C, Cannella D. Laccase-derived lignin compounds boost cellulose oxidative enzymes AA9. Biotechnol Biofuels. 2018;11(1):10.
Zhao C, Xie S, Pu Y, Zhang R, Huang F, Ragauskas AJ, et al. Synergistic enzymatic and microbial lignin conversion. Green Chem. 2016;18(5):1306–12.
Kersten PJ, Tien M, Kalyanaraman B, Kirk TK. The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. J Biol Chem. 1985;260(5):2609–12.
Hammel KE, Jensen KA, Mozuch MD, Landucci LL, Tien M, Pease EA. Ligninolysis by a purified lignin peroxidase. J Biol Chem. 1993;268(17):12274–81.
Abdel-Hamid AM, Solbiati JO, Cann IKO. Chapter one—insights into lignin degradation and its potential industrial applications. Adv Appl Microbiol. 2013;82:1–28. https://doi.org/10.1016/B978-0-12-407679-2.00001-6.
Ten Have R, Rietjens IM, Hartmans S, Swarts HJ, Field JA. Calculated ionisation potentials determine the oxidation of vanillin precursors by lignin peroxidase. FEBS Lett. 1998;430(3):390–2.
Banci L, Ciofi-Baffoni S, Tien M. Lignin and Mn peroxidase-catalyzed oxidation of phenolic lignin oligomers. Biochemistry. 1999;38(10):3205–10.
Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microb Technol. 2002;30(4):454–66. https://doi.org/10.1016/S0141-0229(01)00528-2.
Huang XF, Santhanam N, Badri DV, Hunter WJ, Manter DK, Decker SR, et al. Isolation and characterization of lignin-degrading bacteria from rainforest soils. Biotechnol Bioeng. 2013;110(6):1616–26.
Hilden K, Makela MR, Steffen KT, Hofrichter M, Hatakka A, Archer DB, et al. Biochemical and molecular characterization of an atypical manganese peroxidase of the litter-decomposing fungus Agrocybe praecox. Fungal Genet Biol. 2014;72:131–6.
Martınez AT. Molecular biology and structure-function of lignin-degrading heme peroxidases. Enzyme Microb Technol. 2002;30(4):425–44.
Heinfling A, Martı́nez MJ, Martı́nez AT, Bergbauer M, Szewzyk U. Purification and characterization of peroxidases from the dye-decolorizing fungus Bjerkandera adusta. FEMS Microbiol Lett. 1998;165(1):43–50.
Van Bloois E, Pazmino DET, Winter RT, Fraaije MW. A robust and extracellular heme-containing peroxidase from Thermobifida fusca as prototype of a bacterial peroxidase superfamily. Appl Microbiol Biotechnol. 2010;86(5):1419–30.
Linde D, Coscolin C, Liers C, Hofrichter M, Martinez AT, Ruiz-Duenas FJ. Heterologous expression and physicochemical characterization of a fungal dye-decolorizing peroxidase from Auricularia auricula-judae. Protein Expr Purif. 2014;103:28–37.
Rahmanpour R, Bugg TD. Characterisation of Dyp-type peroxidases from Pseudomonas fluorescens Pf-5: oxidation of Mn (II) and polymeric lignin by Dyp1B. Arch Biochem Biophys. 2015;574:93–8.
Brown ME, Barros T, Chang MC. Identification and characterization of a multifunctional dye peroxidase from a lignin-reactive bacterium. ACS Chem Biol. 2012;7(12):2074–81.
Ahmad M, Roberts JN, Hardiman EM, Singh R, Eltis LD, Bugg TD. Identification of DypB from Rhodococcus jostii RHA1 as a lignin peroxidase. Biochemistry. 2011;50(23):5096–107.
Lin L, Cheng Y, Pu Y, Sun S, Li X, Jin M, et al. Systems biology-guided biodesign of consolidated lignin conversion. Green Chem. 2016;18(20):5536–47.
De Gonzalo G, Colpa DI, Habib MHM, Fraaije MW. Bacterial enzymes involved in lignin degradation. J Biotechnol. 2016;236:110–9. https://doi.org/10.1016/j.jbiotec.2016.08.011.
Kirk TK, Farrell RL. Enzymatic" combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41(1):465–501.
Shi Y, Chai L, Tang C, Yang Z, Zhang H, Chen R, et al. Characterization and genomic analysis of kraft lignin biodegradation by the beta-proteobacterium Cupriavidus basilensis B-8. Biotechnol Biofuels. 2013;6(1):1.
Chen Y, Chai L, Zhu Y, Yang Z, Zheng Y, Zhang H. Biodegradation of kraft lignin by a bacterial strain Comamonas sp. B-9 isolated from eroded bamboo slips. J Appl Microbiol. 2012;112(5):900–6.
Jensen KA, Houtman CJ, Ryan ZC, Hammel KE. Pathways for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microbiol. 2001;67(6):2705–11.
Kent MS, Zeng J, Rader N, Avina IC, Simoes CT, Brenden CK, et al. Efficient conversion of lignin into a water-soluble polymer by a chelator-mediated Fenton reaction: optimization of H2O2 use and performance as a dispersant. Green Chem. 2018;20(13):3024–37.
Sun Y, Yang S, Li G, Li M. Preparation of starch phosphate carbamides and its application for improvement of noodle quality. Czech J Food Sci. 2019;37(6):456–62.
Li X, Zheng Y. Lignin-enzyme interaction: mechanism, mitigation approach, modeling, and research prospects. Biotechnol Adv. 2017;35(4):466–89. https://doi.org/10.1016/j.biotechadv.2017.03.010.
Tomizawa S, Chuah J-A, Matsumoto K, Doi Y, Numata K. Understanding the limitations in the biosynthesis of polyhydroxyalkanoate (PHA) from lignin derivatives. ACS Sustain Chem Eng. 2014;2(5):1106–13.
Sannigrahi P, Ragauskas AJ, Miller SJ. Lignin structural modifications resulting from ethanol organosolv treatment of loblolly pine. Energy Fuels. 2009;24(1):683–9.
Sato Y, Moriuchi H, Hishiyama S, Otsuka Y, Oshima K, Kasai D, et al. Identification of three alcohol dehydrogenase genes involved in the stereospecific catabolism of arylglycerol-β-aryl ether by Sphingobium sp. strain SYK-6. Appl Environ Microbiol. 2009;75(16):5195–201.
Reiter J, Strittmatter H, Wiemann LO, Schieder D, Sieber V. Enzymatic cleavage of lignin β-O-4 aryl ether bonds via net internal hydrogen transfer. Green Chem. 2013;15(5):1373–81.
Gibson DT, Parales RE. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr Opin Biotechnol. 2000;11(3):236–43.
Lee J-H, Wendisch VF. Biotechnological production of aromatic compounds of the extended shikimate pathway from renewable biomass. J Biotechnol. 2017;257:211–21.
Kutsuki H, Gold MH. Generation of hydroxyl radical and its involvement in lignin degradation by Phanerochaete chrysosporium. BBRC. 1982;109(2):320–7.
Kamaya Y, Nakatsubo F, Higuchi T, Iwahara S. Degradation of D, l-syringaresinol, a β-β′-linked lignin model compound, by Fusarium solani: M-13-1. Arch Microbiol. 1981;129:305–9.
Masai E, Harada K, Peng X, Kitayama H, Katayama Y, Fukuda M. Cloning and characterization of the ferulic acid catabolic genes of Sphingomonas paucimobilis SYK-6. Appl Environ Microbiol. 2002;68(9):4416–24.
Prim N, Pastor F, Diaz P. Biochemical studies on cloned Bacillus sp. BP-7 phenolic acid decarboxylase PadA. Appl Microbiol Biotechnol. 2003;63(1):51–6.
Godoy L, Martínez C, Carrasco N, Ganga MA. Purification and characterization of a p-coumarate decarboxylase and a vinylphenol reductase from Brettanomyces bruxellensis. Int J Food Microbiol. 2008;127(1–2):6–11.
Rodriguez A, Martnez JA, Flores N, Escalante A, Gosset G, Bolivar F. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds. Microb Cell Fact. 2014;13(1):126.
Cluis CP, Ekins A, Narcross L, Jiang H, Gold ND, Burja AM, et al. Identification of bottlenecks in Escherichia coli engineered for the production of CoQ10. Metab Eng. 2011;13(6):733–44.
Curran KA, Leavitt JM, Karim AS, Alper HS. Metabolic engineering of muconic acid production in Saccharomyces cerevisiae. Metab Eng. 2013;15:55–66.
Gallage NJ, Moller BL. Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol Plant. 2015;8(1):40–57.
Grandy AS, Neff JC, Weintraub MN. Carbon structure and enzyme activities in alpine and forest ecosystems. Soil Biol Biochem. 2007;39(11):2701–11.
Harwood CS, Parales RE. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50(1):553–90.
Kamimura N, Masai E. The protocatechuate 4, 5-cleavage pathway: overview and new findings. In: Nojiri H, Tsuda M, Fukuda M, Kamagata Y, editors. Biodegradative bacteria. Japan: Springer; 2014. p. 207–226.
Villar J, Caperos A, Garcia-Ochoa F. Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant. Wood Sci Technol. 2001;35(3):245–55.
Varanasi P, Singh P, Auer M, Adams PD, Simmons BA, Singh S. Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol Biofuels. 2013;6(1):14.
Kang S, Li X, Fan J, Chang J. Hydrothermal conversion of lignin: a review. Renew Sustain Energy Rev. 2013;27:546–58.
Araujo JD, Grande CA, Rodrigues AE. Vanillin production from lignin oxidation in a batch reactor. Chem Eng Res Des. 2010;88(8):1024–32.
Bugg TD, Ahmad M, Hardiman EM, Singh R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol. 2011;22(3):394–400.
Rady A, Ekramirad N, Adedeji A, Li M, Alimardani R. Hyperspectral imaging for detection of codling moth infestation in GoldRush apples. Postharvest Biol Technol. 2017;129:37–44.
Dias F, Gomez JGC, Silva LFD. Exploring the microbial production of aromatic fine chemicals to overcome the barriers of traditional methods. Adv Appl Sci Res. 2017;8(1):94–109.
Weitkamp P, Vosmann K, Weber N. Highly efficient preparation of lipophilic hydroxycinnamates by solvent-free lipase-catalyzed transesterification. J Agric Food Chem. 2006;54(19):7062–8.
Kartal M, Yildiz S, Kaya S, Kurucu S, Topcu G. Antimicrobial activity of propolis samples from two different regions of Anatolia. J Ethnopharmacol. 2003;86(1):69–73.
Li M, Zhang F, Liu Z, Guo X, Wu Q, Qiao L. Controlled release system by active gelatin film incorporated with β-cyclodextrin-thymol inclusion complexes. Food Bioprocess Technol. 2018;11(9):1695–702.
Li M, Ye R. Edible active packaging for food application: materials and technology. In: Masuelli MA, editor. Biopackaging. 1st ed. Boca Raton: CRC Press; 2017. p. 1.
Li C, Zhao X, Wang A, Huber GW, Zhang T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev. 2015;115(21):11559–624.
Isikgor FH, Becer CR. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem. 2015;6(25):4497–559.
Kumar N, Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnol Rep. 2014;4:86–93.
Javaheri M, Dadar A, Babaeian M. Effect of salicylic acid spray in seedling stage on yield and yield components of tomato. J Appl Sci Agric. 2014;9(3):924–8.
Li M, Ekramirad N, Rady A, Adedeji A. Application of acoustic emission and machine learning to detect codling moth infested apples. Trans ASABE. 2018;61(3):1157–64.
Fujita T, Nguyen HD, Ito T, Zhou S, Osada L, Tateyama S, et al. Microbial monomers custom-synthesized to build true bio-derived aromatic polymers. Appl Microbiol Biotechnol. 2013;97(20):8887–94.
Tsuji H, Matsuoka H, Itsuno S. Synthesis, physical properties, and crystallization of optically active poly (l-phenyllactic acid) and poly (l-phenyllactic acid-co-l-lactic acid). J Appl Polym Sci. 2008;110(6):3954–62.
Billingsley JM, DeNicola AB, Tang Y. Technology development for natural product biosynthesis in Saccharomyces cerevisiae. Curr Opin Biotechnol. 2016;42:74–83.
Liu X, Lin J, Hu H, Zhou B, Zhu B. De novo biosynthesis of resveratrol by site-specific integration of heterologous genes in Escherichia coli. FEMS Microbiol Lett. 2016;363(8):fnw061.
Shen X-H, Zhou N-Y, Liu S-J. Degradation and assimilation of aromatic compounds by Corynebacterium glutamicum: another potential for applications for this bacterium? Appl Microbiol Biotechnol. 2012;95(1):77–89.
Bugg TD. Dioxygenase enzymes: catalytic mechanisms and chemical models. Tetrahedron. 2003;59(36):7075–101.
Harwood CS, Nichols NN, Kim M-K, Ditty JL, Parales RE. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176(21):6479–88.
Johnson CW, Beckham GT. Aromatic catabolic pathway selection for optimal production of pyruvate and lactate from lignin. Metab Eng. 2015;28:240–7.
Hopwood DA, Chater KF. Genetics of bacterial diversity. Cambridge: Academic Press; 2012.
Zhang R, Li C, Wang J, Yan Y. Microbial ligninolysis: toward a bottom-up approach for lignin upgrading. Biochemistry. 2018;58:1501–10.
Wells T Jr, Ragauskas AJ. Biotechnological opportunities with the β-ketoadipate pathway. Trends Biotechnol. 2012;30(12):627–37.
Chen X, Kohl TA, Rückert C, Rodionov DA, Li L-H, Ding J-Y, et al. Phenylacetic acid catabolism and transcriptional regulation in Corynebacterium glutamicum. Appl Environ Microbiol. 2012;78:5796–804.
Teufel R, Mascaraque V, Ismail W, Voss M, Perera J, Eisenreich W, et al. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. PNAS. 2010;107(32):14390–5.
Teufel R, Gantert C, Voss M, Eisenreich W, Haehnel W, Fuchs G. Studies on the mechanism of ring-hydrolysis in phenylacetate degradation-a metabolic branching point. J Biol Chem. 2011;286:11021–34.
Polen T, Spelberg M, Bott M. Toward biotechnological production of adipic acid and precursors from biorenewables. J Biotechnol. 2013;167(2):75–84.
Sengupta S, Jonnalagadda S, Goonewardena L, Juturu V. Metabolic engineering of a novel muconic acid biosynthetic pathway via 4-hydroxybenzoic acid in Escherichia coli. Appl Environ Microbiol. 2015;81:8037–43.
Van Duuren J, Brehmer B, Mars A, Eggink G, Dos Santos VM, Sanders J. A limited LCA of bio-adipic acid: manufacturing the nylon-6, 6 precursor adipic acid using the benzoic acid degradation pathway from different feedstocks. Biotechnol Bioeng. 2011;108(6):1298–306.
Shi Y, Yan X, Li Q, Wang X, Xie S, Chai L, et al. Directed bioconversion of kraft lignin to polyhydroxyalkanoate by Cupriavidus basilensis B-8 without any pretreatment. Process Biochem. 2017;52:238–42.
Li X, Shi J, Das L, Tharayil N, Zheng Y. A novel platform for bioupgrading of lignin to valuable nutraceuticals and pharmaceuticals. In: 2018 ASABE Annual International Meeting; St. Joseph, MI: ASABE; 2018. p. 1.
Nogales J, García J, Díaz E. Degradation of aromatic compounds in Pseudomonas: A systems biology view. Aerobic utilization of hydrocarbons, oils and lipids. New York: Springer; 2017. pp. 1–49.
Tinikul R, Chenprakhon P, Maenpuen S, Chaiyen P. Biotransformation of plant-derived phenolic acids. Biotechnol J. 2018;13(6):1700632.
Liu Z-H, Xie S, Lin F, Jin M, Yuan JS. Combinatorial pretreatment and fermentation optimization enabled a record yield on lignin bioconversion. Biotechnol Biofuels. 2018;11(1):21.
Gall DL, Kontur WS, Lan W, Kim H, Li Y, Ralph J, et al. In vitro enzymatic depolymerization of lignin with release of syringyl, guaiacyl, and tricin units. Appl Environ Microbiol. 2018;84(3):e02076–e2117.
Cybulska I, Brudecki G, Rosentrater K, Julson JL, Lei H. Comparative study of organosolv lignin extracted from prairie cordgrass, switchgrass and corn stover. Bioresour Technol. 2012;118:30–6.
Fasching M, Schroder P, Wollboldt RP, Weber HK, Sixta H. A new and facile method for isolation of lignin from wood based on complete wood dissolution. Holz. 2008;62(1):15–23.
Eckert C, Liotta C, Ragauskas A, Hallett J, Kitchens C, Hill E, et al. Tunable solvents for fine chemicals from the biorefinery. Green Chem. 2007;9(6):545–8.
Zakzeski J, Jongerius AL, Weckhuysen BM. Transition metal catalyzed oxidation of Alcell lignin, soda lignin, and lignin model compounds in ionic liquids. Green Chem. 2010;12(7):1225–366.
Brzonova I, Asina F, Andrianova AA, Kubatova A, Smoliakova IP, Kozliak EI, et al. Fungal biotransformation of insoluble kraft lignin into a water soluble polymer. Ind Eng Chem Res. 2017;56(21):6103–13.
Stevens JC, Das L, Mobley JK, Asare SO, Lynn BC, Rodgers DW, et al. Understanding laccase-ionic liquid interactions toward biocatalytic lignin conversion in aqueous ionic liquids. ACS Sustain Chem Eng. 2019;7:15928–38.
Stevens JC, Shi J. Biocatalysis in ionic liquids for lignin valorization: Opportunities and recent developments. Biotechnol Adv. 2019. https://doi.org/10.1016/j.biotechadv.2019.107418.
Lv Y, Chen Y, Sun S, Hu Y. Interaction among multiple microorganisms and effects of nitrogen and carbon supplementations on lignin degradation. Bioresour Technol. 2014;155:144–51.
Dietrich D, Illman B, Crooks C. Differential sensitivity of polyhydroxyalkanoate producing bacteria to fermentation inhibitors and comparison of polyhydroxybutyrate production from Burkholderia cepacia and Pseudomonas pseudoflava. BMC Res Notes. 2013;6(1):219.
Jonsson LJ, Martin C. Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol. 2016;199:103–12.
Larsson S, Reimann A, Nilvebrant N-O, Jonsson LJ. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol. 1999;77(1–3):91–103.
Van Duuren JB, Wijte D, Karge B, Martins dos Santos VA, Yang Y, Mars AE, et al. pH-stat fed-batch process to enhance the production of cis, cis-muconate from benzoate by Pseudomonas putida KT2440-JD1. Biotechnol Prog. 2012;28(1):85–92.
Berezina N, Yada B, Lefebvre R. From organic pollutants to bioplastics: insights into the bioremediation of aromatic compounds by Cupriavidus necator. N Biotechnol. 2015;32(1):47–53. https://doi.org/10.1016/j.nbt.2014.09.003.
Wang W, Yang S, Hunsinger GB, Pienkos PT, Johnson DK. Connecting lignin-degradation pathway with pre-treatment inhibitor sensitivity of Cupriavidus necator. Front Microbiol. 2014;5:247.
Dietrich K, Dumont M-J, Schwinghamer T, Orsat V, Del Rio LF. Model study to assess softwood hemicellulose hydrolysates as the carbon source for PHB production in Paraburkholderia sacchari IPT 101. Biomacromol. 2017;19(1):188–200.
Wang Y, Liu Q, Yan L, Gao Y, Wang Y, Wang W. A novel lignin degradation bacterial consortium for efficient pulping. Bioresour Technol. 2013;139:113–9.
Yadav S, Chandra R. Syntrophic co-culture of Bacillus subtilis and Klebsiella pneumonia for degradation of kraft lignin discharged from rayon grade pulp industry. JEnvS. 2015;33:229–38.
Ramachandra M, Crawford DL, Hertel G. Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl Environ Microbiol. 1988;54(12):3057–63.
Xu R, Zhang K, Liu P, Han H, Zhao S, Kakade A, et al. Lignin depolymerization and utilization by bacteria. Bioresour Technol. 2018;269:557–66. https://doi.org/10.1016/j.biortech.2018.08.118.
Ding W, Si M, Zhang W, Zhang Y, Chen C, Zhang L, et al. Functional characterization of a vanillin dehydrogenase in Corynebacterium glutamicum. Sci Rep. 2015;5:8044.
Davis JR, Sello JK. Regulation of genes in Streptomyces bacteria required for catabolism of lignin-derived aromatic compounds. Appl Microbiol Biotechnol. 2010;86(3):921–9.
Li X, Zheng Y. Biotransformation of lignin: mechanisms, applications and future work. Biotechnol Prog. 2019.
Liu Z-H, Le RK, Kosa M, Yang B, Yuan J, Ragauskas AJ. Identifying and creating pathways to improve biological lignin valorization. Renew Sustain Energy Rev. 2019;105:349–62. https://doi.org/10.1016/j.rser.2019.02.009.
Fang X, Li Q, Lin Y, Lin X, Dai Y, Guo Z, et al. Screening of a microbial consortium for selective degradation of lignin from tree trimmings. Bioresour Technol. 2018;254:247–55.
Kuhad RC, Gupta R, Singh A. Microbial cellulases and their industrial applications. Enzyme Res. 2011.
Shin SK, Ko YJ, Hyeon JE, Han SO. Studies of advanced lignin valorization based on various types of lignolytic enzymes and microbes. Bioresour Technol. 2019;289:121728. https://doi.org/10.1016/j.biortech.2019.121728.
Lopez-Mondejar R, Algora C, Baldrian P. Lignocellulolytic systems of soil bacteria: a vast and diverse toolbox for biotechnological conversion processes. Biotechnol Adv. 2019;37(6):107374. https://doi.org/10.1016/j.biotechadv.2019.03.013.
Tian J-H, Pourcher A-M, Bouchez T, Gelhaye E, Peu P. Occurrence of lignin degradation genotypes and phenotypes among prokaryotes. Appl Microbiol Biotechnol. 2014;98(23):9527–44.
Xie S, Sun Q, Pu Y, Lin F, Sun S, Wang X, et al. Advanced chemical design for efficient lignin bioconversion. ACS Sustain Chem Eng. 2017;5(3):2215–23.
Longe LF, Couvreur J, Leriche Grandchamp M, Garnier G, Allais F, Saito K. Importance of mediators for lignin degradation by fungal laccase. ACS Sustain Chem Eng. 2018;6(8):10097–10707.
Li M, Wilkins M. Fed-batch cultivation and adding supplements to increase yields of polyhydroxybutyrate production by Cupriavidus necator from corn stover alkaline pretreatment liquor. Bioresour Technol. 2020;299:122676.
Xie N-Z, Liang H, Huang R-B, Xu P. Biotechnological production of muconic acid: current status and future prospects. Biotechnol Adv. 2014;32(3):615–22.
Li M, Wijewardane NK, Ge Y, Xu Z, Wilkins MR. Visible/near infrared spectroscopy and machine learning for predicting polyhydroxybutyrate production cultured on alkaline pretreated liquor from corn stover. Bioresour Technol Rep. 2020;9:100386. https://doi.org/10.1016/j.biteb.2020.100386.
Li M, Wilkins M. Flow cytometry for quantitation of polyhydroxybutyrate production by Cupriavidus necator using alkaline pretreated liquor from corn stover. Bioresour Technol. 2020;295:122254. https://doi.org/10.1016/j.biortech.2019.122254.
Sainsbury PD, Mineyeva Y, Mycroft Z, Bugg TDH. Chemical intervention in bacterial lignin degradation pathways: development of selective inhibitors for intradiol and extradiol catechol dioxygenases. Bioorg Chem. 2015;60:102–9. https://doi.org/10.1016/j.bioorg.2015.05.002.
Raj A, Reddy MK, Chandra R. Identification of low molecular weight aromatic compounds by gas chromatography–mass spectrometry (GC–MS) from kraft lignin degradation by three Bacillus sp. Int Biodeterior Biodegrad. 2007;59(4):292–6.
Crawford DL, Pometto AL, Crawford RL. Lignin degradation by Streptomyces viridosporus: isolation and characterization of a new polymeric lignin degradation intermediate. Appl Environ Microbiol. 1983;45(3):898–904.
Pometto AL, Crawford DL. Catabolic fate of Streptomyces viridosporus T7A-produced, acid-precipitable polymeric lignin upon incubation with ligninolytic Streptomyces species and Phanerochaete chrysosporium. Appl Environ Microbiol. 1986;51(1):171–9.
Chen Y, Chai L, Tang C, Yang Z, Zheng Y, Shi Y, et al. Kraft lignin biodegradation by Novosphingobium sp. B-7 and analysis of the degradation process. Bioresour Technol. 2012;123:682–5.
Kumar M, Singh J, Singh MK, Singhal A, Thakur IS. Investigating the degradation process of kraft lignin by β-proteobacterium, Pandoraea sp. ISTKB Environ Sci Pollut Res. 2015;22(20):15690–702.
Koncsag CI, Eastwood D, Collis AE, Coles SR, Clark AJ, Kirwan K, et al. Extracting valuable compounds from straw degraded by Pleurotus ostreatus. Resour Conserv Recycl. 2012;59:14–22.
Xie S, Qin X, Cheng Y, Laskar D, Qiao W, Sun S, et al. Simultaneous conversion of all cell wall components by an oleaginous fungus without chemi-physical pretreatment. Green Chem. 2015;17(3):1657–67.
Raj A, Chandra R, Reddy MMK, Purohit HJ, Kapley A. Biodegradation of kraft lignin by a newly isolated bacterial strain, Aneurinibacillus aneurinilyticus from the sludge of a pulp paper mill. World J Microbiol Biotechnol. 2007;23(6):793–9.
Chong G-G, Huang X-J, Di J-H, Xu D-Z, He Y-C, Pei Y-N, et al. Biodegradation of alkali lignin by a newly isolated Rhodococcus pyridinivorans CCZU-B16. Bioprocess Biosyst Eng. 2018;41(4):501–10.
Zamzuri N, Abd-Aziz S, Rahim R, Phang L, Alitheen N, Maeda T. A rapid colorimetric screening method for vanillic acid and vanillin-producing bacterial strains. J Appl Microbiol. 2014;116(4):903–10.
Graf N, Altenbuchner J. Genetic engineering of Pseudomonas putida KT2440 for rapid and high-yield production of vanillin from ferulic acid. Appl Microbiol Biotechnol. 2014;98(1):137–49.
Shi Y, Chai L, Tang C, Yang Z, Zheng Y, Chen Y, et al. Biochemical investigation of kraft lignin degradation by Pandoraea sp. B-6 isolated from bamboo slips. Bioprocess Biosyst Eng. 2013;36(12):1957–65.
Krammer G, Ley JP, Geißler K, Geißler T, Gomoll F, Welters P et al. Method for biotechnological production of methylized cinnamic acids and cinnamic acid esters, methylized phenethylamines and the coupling products thereof, particularly of cinnamic acid amides. Google Patents; 2016.
Okamura-Abe Y, Abe T, Nishimura K, Kawata Y, Sato-Izawa K, Otsuka Y, et al. Beta-ketoadipic acid and muconolactone production from a lignin-related aromatic compound through the protocatechuate 3,4-metabolic pathway. J Biosci Bioeng. 2016;121(6):652–8. https://doi.org/10.1016/j.jbiosc.2015.11.007.
Johnson CW, Salvachua D, Khanna P, Smith H, Peterson DJ, Beckham GT. Enhancing muconic acid production from glucose and lignin-derived aromatic compounds via increased protocatechuate decarboxylase activity. Metab Eng Commun. 2016;3:111–9.
Otsuka Y, Nakamura M, Shigehara K, Sugimura K, Masai E, Ohara S, et al. Efficient production of 2-pyrone 4, 6-dicarboxylic acid as a novel polymer-based material from protocatechuate by microbial function. Appl Microbiol Biotechnol. 2006;71(5):608–14.
Qian Y, Otsuka Y, Sonoki T, Mukhopadhyay B, Nakamura M, Jellison J, et al. Engineered microbial production of 2-pyrone-4, 6-dicarboxylic acid from lignin residues for use as an industrial platform chemical. BioResources. 2016;11(3):6097–109.
Hong C-Y, Ryu S-H, Jeong H, Lee S-S, Kim M, Choi I-G. Phanerochaete chrysosporium multienzyme catabolic system for in vivo modification of synthetic lignin to succinic acid. ACS Chem Biol. 2017;12(7):1749–59.
Kohlstedt M, Starck S, Barton N, Stolzenberger J, Selzer M, Mehlmann K, et al. From lignin to nylon: cascaded chemical and biochemical conversion using metabolically engineered Pseudomonas putida. Metab Eng. 2018;47:279–93. https://doi.org/10.1016/j.ymben.2018.03.003.
Salvachúa D, Johnson CW, Singer CA, Rohrer H, Peterson DJ, Black BA, et al. Bioprocess development for muconic acid production from aromatic compounds and lignin. Green Chem. 2018;20(21):5007–199.
Barton N, Horbal L, Starck S, Kohlstedt M, Luzhetskyy A, Wittmann C. Enabling the valorization of guaiacol-based lignin: integrated chemical and biochemical production of cis, cis-muconic acid using metabolically engineered Amycolatopsis sp ATCC 39116. Metab Eng. 2018;45:200–10. https://doi.org/10.1016/j.ymben.2017.12.001.
Becker J, Kuhl M, Kohlstedt M, Starck S, Wittmann C. Metabolic engineering of Corynebacterium glutamicum for the production of cis, cis-muconic acid from lignin. Microb Cell Fact. 2018;17(1):115.
Mycroft Z, Gomis M, Mines P, Law P, Bugg TD. Biocatalytic conversion of lignin to aromatic dicarboxylic acids in Rhodococcus jostii RHA1 by re-routing aromatic degradation pathways. Green Chem. 2015;17(11):4974–9.
Energy USDo. U.S Department of Energy Genome Programs image gallery. U.S Department of Energy. 2019. Accessed 19 Oct 2019.
Breunig M. Lignin—a natural resource with huge potential. BIOPRO Baden-Württemberg, University of Freiburg. 2017. Accessed 19 Oct 2019.
Ojumu T, Yu J, Solomon B. Production of polyhydroxyalkanoates, a bacterial biodegradable polymers. Afr J Biotechnol. 2004;3(1):18–24.
Sigurdsson AF. The Omega-3 Index. Doc's opinion. 2015. Accessed 13 Oct 2019.
Acknowledgements
The authors would like to acknowledge the Nebraska Agricultural Research Division for providing funding for the authors of this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, M., Wilkins, M. Lignin bioconversion into valuable products: fractionation, depolymerization, aromatic compound conversion, and bioproduct formation. Syst Microbiol and Biomanuf 1, 166–185 (2021). https://doi.org/10.1007/s43393-020-00016-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-020-00016-6