Abstract
Purpose The pomegranate juice manufacturing industry produces vast quantities of non-edible portions of fruit as a by-product. The rind is a good source of many beneficial functional components, especially polyphenols and in particular tannins. This research was undertaken to expose the presence of active substances, including tannin compounds, in the crude extracts (aqueous (AE) and methanolic (ME)) and their fractions increasing in polarity, from Moroccan pomegranate rind samples.
Methods To describe the molecular distribution of tannin content in crude extracts, steric exclusion chromatography (SEC) on Sephadex gel 50 was carried out. In addition, their antimicrobial properties were tested using agar diffusion, dispersion, and microdilution methods against the bacterial strains Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and the yeast strains Saccharomyces cerevisiae and Candida tropicalis.
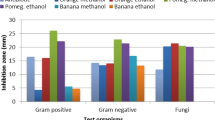
Results For both crude extracts, the SEC showed qualitative and quantitative differences in tannin polymerization. Diameters of inhibition zones (DIZ) obtained against bacterial species ranged from 20.6 to 30.3 mm for ME, from 10.8 to 15.3 mm for AE and from 0 to 16.6 mm for fractions. Among the selected fungal cultures, the highest antifungal activity was reported against S. cerevisiae; with an inhibition rate (I %) ranging up to 99.00% for AE. With I % reaching up to 93.35%, the C. tropicalis strain was more sensitive to ME, although the AE has no inhibitory effect on this yeast. For fractions, the I % ranged from 7.03 to 98.03% where a synergistic antifungal effect was observed between fractions.
Conclusion Pomegranate processing by-product is a potential source which could be used as natural preservatives in food industry and non-toxic matrices replacing hazardous materials in surface treatment.
Graphic Abstract








Similar content being viewed by others
Abbreviations
- PRP:
-
Pomegranate rind powder
- DM:
-
Dry matter
- ME:
-
Methanolic extract
- AE:
-
Aqueous extract
- HF:
-
Hexane fraction
- CF:
-
Chloroform fraction
- BF:
-
Butanol fraction from ME
- EAF:
-
Ethyl acetate fraction
- BF′:
-
Butanol fraction from AE
- MIC:
-
Minimal inhibitory concentration
References
Jiménez-Moreno, N., Esparza, I., Bimbela, F., Gandía, L.M., Ancín-Azpilicueta, C.: Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 50, 1–48 (2020). https://doi.org/10.1080/10643389.2019.1694819
Banerjee, J., Singh, R., Vijayaraghavan, R., MacFarlane, D., Patti, A.F., Arora, A.: Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 225, 10–22 (2017). https://doi.org/10.1016/j.foodchem.2016.12.093
Laufenberg, G., Kunz, B., Nystroem, M.: Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour. Technol. 87, 167–198 (2003). https://doi.org/10.1016/S0960-8524(02)00167-0
Parfitt, J., Barthel, M., Macnaughton, S.: Food waste within food supply chains: quantification and potential for change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 365, 3065–3081 (2010). https://doi.org/10.1098/rstb.2010.0126
Wu, V.C.-H., Qiu, X., Bushway, A., Harper, L.: Antibacterial effects of American cranberry (Vaccinium macrocarpon) concentrate on foodborne pathogens. LWT - Food Sci. Technol. 41, 1834–1841 (2008). https://doi.org/10.1016/j.lwt.2008.01.001
Sharif, M.K., Khalid, R.: Chapter 1–Nutraceuticals: Myths Versus Realities. In: Holban, A.M., Grumezescu, A.M. (eds.) Therapeutic Foods, pp. 3–21. Academic Press, Cambridge (2018)
Qu, W., Pan, Z., Zhang, R., Ma, H., Chen, X., Zhu, B., Wang, Z., Atungulu, G.G.: Integrated Extraction and Anaerobic Digestion Process for Recovery of Nutraceuticals and Biogas from Pomegranate Marc. Trans. ASABE. 52, 1997–2006 (2009). https://doi.org/10.13031/2013.29196
Talekar, S., Patti, A.F., Singh, R., Vijayraghavan, R., Arora, A.: From waste to wealth: High recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crops Prod. 112, 790–802 (2018). https://doi.org/10.1016/j.indcrop.2017.12.023
Ashton, R.W.: The incredible pomegranate: plant and fruit. Third Millennium Publishing, Tempe, AZ (2006)
Macheix, J.-J., Fleuriet, A., Jay-Allemand, C.: Les composés phénoliques des végétaux: un exemple de métabolites secondaires d’importance économique. PPUR presses polytechniques, Lausanne (2005)
Lairini, S., Bouslamti, R., Zerrouq, F.: Enhancement of the aqueous extract of the bark of Punica granatum fruit through the study of its antimicrobial and antioxidant activities. J Mater Environ Sci. 5, 2314–2318 (2014)
Braga, L.C., Leite, A.A.M., Xavier, K.G.S., Takahashi, J.A., Bemquerer, M.P., Chartone-Souza, E., Nascimento, A.M.A.: Synergic interaction between pomegranate extract and antibiotics against Staphylococcus aureus. Can. J. Microbiol. 51, 541–547 (2005). https://doi.org/10.1139/w05-022
Loizzo, M.R., Aiello, F., Tenuta, M.C., Leporini, M., Falco, T., Tundis, R.: Pomegranate (Punica granatum L,). In: Nabavi, S.M., Silva, A.S. (eds.) Nonvitamin and Nonmineral Nutritional Supplements, Chapter 3.46, pp. 467–472. Academic Press, Cambridge (2019)
Malik, A., Afaq, F., Sarfaraz, S., Adhami, V.M., Syed, D.N., Mukhtar, H.: Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl. Acad. Sci. 102, 14813–14818 (2005). https://doi.org/10.1073/pnas.0505870102
Akesbi, N.: Une nouvelle stratégie pour l’agriculture marocaine: Le «Plan Maroc Vert». New Medit: Mediterranean Journal of Economics, Agriculture and Environment = Revue Méditerranéenne dʹEconomie Agriculture et Environment. 11, 12 (2012)
Ismail, T., Akhtar, R., Riaz, M., Ismail, A.: Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies. Int. J. Food Sci. Nutr. 65, 661–666 (2014). https://doi.org/10.3109/09637486.2014.908170
Tzulker, R., Glazer, I., Bar-Ilan, I., Holland, D., Aviram, M., Amir, R.: Antioxidant Activity, Polyphenol Content, and Related Compounds in Different Fruit Juices and Homogenates Prepared from 29 Different Pomegranate Accessions. J. Agric. Food Chem. 55, 9559–9570 (2007). https://doi.org/10.1021/jf071413n
Ben-Ali, S., Akermi, A., Mabrouk, M., Ouederni, A.: Optimization of extraction process and chemical characterization of pomegranate peel extract. Chem. Pap. 72, 2087–2100 (2018). https://doi.org/10.1007/s11696-018-0427-5
Lansky, E.P., Newman, R.A.: Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 109, 177–206 (2007). https://doi.org/10.1016/j.jep.2006.09.006
Xi, J., He, L., Yan, L.: Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 230, 354–361 (2017). https://doi.org/10.1016/j.foodchem.2017.03.072
Qu, W., Pan, Z., Ma, H.: Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 99, 16–23 (2010). https://doi.org/10.1016/j.jfoodeng.2010.01.020
Growther, L.: Antibacterial activity of punica granatum peel extracts against shiga toxin producing e. COLI. Pharm Res. 1, 9 (2012)
Aziz, Md.A.: Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos paniculata barks and fruits. J. Integr. Med. 13, 173–184 (2015). https://doi.org/10.1016/S2095-4964(15)60179-0
Raaman, N.: Phytochemical Techniques. New India Publishing Agency, Pitam Pura, New Delhi (2006)
Firdouse, S., Alam, P.: Phytochemical investigation of extract of Amorphophallus campanulatus tubers. International Journal of Phytomedicine. 3, 32–35 (2011)
Harborne, A.J.: Phytochemical Methods A Guide to Modern Techniques of Plant Analysis. Springer Science & Business Media, Berlin (1998)
Hossain, M.A., AL-Raqmi, K.A.S., AL-Mijizy, Z.H., Weli, A.M., Al-Riyami, Q.: Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac. J. Trop. Biomed. 3, 705–710 (2013). https://doi.org/10.1016/S2221-1691(13)60142-2
Rigane, H., Chtourou, M., Ben Mahmoud, I., Medhioub, K., Ammar, E.: Polyphenolic compounds progress during olive mill wastewater sludge and poultry manure co-composting, and humic substances building (Southeastern Tunisia). Waste Manag. Res. 33, 73–80 (2015). https://doi.org/10.1177/0734242X14559594
European Committee for Antimicrobial Susceptibility Testing (EUCAST): Determination of minimum inhibitory concentration (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 12, 509–515 (2000). https://doi.org/10.1111/j.1469-0691.2006.01454.x
Li, X., Lu, X., He, Y., Deng, M., Lv, Y.: Identification the Pathogens Causing Rot Disease in Pomegranate (Punica granatum L) in China and the Antifungal Activity of Aqueous Garlic Extract. Forests. 11, 34 (2019). https://doi.org/10.3390/f11010034
Obied, H.K., Allen, M.S., Bedgood, D.R., Prenzler, P.D., Robards, K., Stockmann, R.: Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food Chem. 53, 823–837 (2005). https://doi.org/10.1021/jf048569x
Ismail, T., Sestili, P., Akhtar, S.: Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 143, 397–405 (2012). https://doi.org/10.1016/j.jep.2012.07.004
Kahramanoglu, I., Usanmaz, S.: Pomegranate Production and Marketing. CRC Press, Boco raton (2016)
Redha, A.A.A., Hasan, A.M., Mandeel, Q.: Phytochemical Determinations of Pomegranate (Punica granatum) Rind and Aril Extracts and their Antioxidant, Antidiabetic and Antibacterial Activity. Nat. Prod. Chem. Res. 06, 1–9 (2018). https://doi.org/10.4172/2329-6836.1000332
Al-Zoreky, N.S.: Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 134, 244–248 (2009). https://doi.org/10.1016/j.ijfoodmicro.2009.07.002
Akhtar, S., Ismail, T., Fraternale, D., Sestili, P.: Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 174, 417–425 (2015). https://doi.org/10.1016/j.foodchem.2014.11.035
Zhao, X., Yuan, Z., Fang, Y., et al.: Characterization and evaluation of major anthocyanins in pomegranate (Punica granatum L.) peel of different cultivars and their development phases. Eur Food Res Technol. 236, 109–117 (2013). https://doi.org/10.1007/s00217-012-1869-6
El Houda, N., Douaouri, N.D.: In vivo anti-inflammatory activity and chemical composition of Algerian pomegranate (Punica granatum L.). Int. J. Biosci. IJB. 12, 76–90 (2018). https://doi.org/10.12692/ijb/12.2.76-90
Elfalleh, W., Tlili, N., Nasri, N., Yahia, Y., Hannachi, H., Chaira, N., Ying, M., Ferchichi, A.: Antioxidant Capacities of Phenolic Compounds and Tocopherols from Tunisian Pomegranate (Punica granatum) Fruits. J. Food Sci. 76, C707–C713 (2011). https://doi.org/10.1111/j.1750-3841.2011.02179.x
Farag, R.S., Abdel-Latif, M.S., Emam, S., Tawfeek, S.: Phytochemical screening and polyphenol constituents of pomegranate peels and leave juices. Agriculture and Soil Sciences (LRJASS). 1, 086–093 (2014)
Ahmed, E.: Abdel Moneim: Antioxidant activities of Punica granatum (pomegranate) peel extract on brain of rats. J. Med. Plants Res. 6, 195–199 (2012). https://doi.org/10.5897/JMPR11.500
Orak, H.H., Yagar, H., Isbilir, S.S.: Comparison of antioxidant activities of juice, peel, and seed of pomegranate (Punica granatum L.) and inter-relationships with total phenolic, Tannin, anthocyanin, and flavonoid contents. Food Sci. Biotechnol. 21, 373–387 (2012). https://doi.org/10.1007/s10068-012-0049-6
Masci, A., Coccia, A., Lendaro, E., Mosca, L., Paolicelli, P., Cesa, S.: Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 202, 59–69 (2016). https://doi.org/10.1016/j.foodchem.2016.01.106
Kharchoufi, S., Licciardello, F., Siracusa, L., Muratore, G., Hamdi, M., Restuccia, C.: Antimicrobial and antioxidant features of ‘Gabsiʼ pomegranate peel extracts. Ind. Crops Prod. 111, 345–352 (2018). https://doi.org/10.1016/j.indcrop.2017.10.037
Procópio Gomes, L.A., Alves Figueiredo, L.M., do Rosário Palma, A.L., Corrêa Geraldo, B.M., Isler Castro, K.C., de Oliveira Fugisaki, L.R., Jorge, A.O.C., de Oliveira, L.D., Junqueira, J.C.: Punica granatum L (Pomegranate) Extract In Vivo Study of Antimicrobial Activity against Porphyromonas gingivalis in Galleria mellonella Model. Sci. World J. (2016). https://doi.org/10.1155/2016/8626987
Tian, F., Li, B., Ji, B., Zhang, G., Luo, Y.: Identification and structure–activity relationship of gallotannins separated from Galla chinensis. LWT - Food Sci. Technol. 42, 1289–1295 (2009). https://doi.org/10.1016/j.lwt.2009.03.004
Weissmann, G.: Studies on pine bark extracts. Int. J. Adhes. Adhes. 3, 31–35 (1983). https://doi.org/10.1016/0143-7496(83)90050-7
Çam, M., Hışıl, Y.: Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 123, 878–885 (2010). https://doi.org/10.1016/j.foodchem.2010.05.011
Saad, H., Charrier-El Bouhtoury, F., Pizzi, A., Rode, K., Charrier, B., Ayed, N.: Characterization of pomegranate peels tannin extractives. Ind. Crops Prod. 40, 239–246 (2012). https://doi.org/10.1016/j.indcrop.2012.02.038
Díaz-Mula, H.M., Tomás-Barberán, F.A., García-Villalba, R.: Pomegranate Fruit and Juice (cv. Mollar), Rich in Ellagitannins and Anthocyanins, Also Provide a Significant Content of a Wide Range of Proanthocyanidins. J. Agric. Food Chem. 67, 9160–9167 (2019). https://doi.org/10.1021/acs.jafc.8b07155
Field, J.A., Lettinga, G.: Toxicity of Tannic Compounds to Microorganisms. In: Hemingway, R.W., Laks, P.E. (eds.) Plant Polyphenols, pp. 673–692. Springer, US, Boston, MA (1992)
Rosas-Burgos, E.C., Burgos-Hernández, A., Noguera-Artiaga, L., Kačániová, M., Hernández-García, F., Cárdenas-López, J.L., Carbonell-Barrachina, Á.A.: Antimicrobial activity of pomegranate peel extracts as affected by cultivar: Pomegranate antimicrobial activity. J. Sci. Food Agric. 97, 802–810 (2017). https://doi.org/10.1002/jsfa.7799
Ferrazzano, G.F., Scioscia, E., Sateriale, D., Pastore, G., Colicchio, R., Pagliuca, C., Cantile, T., Alcidi, B., Coda, M., Ingenito, A., Scaglione, E., Cicatiello, A.G., Volpe, M.G., Di Stasio, M., Salvatore, P., Pagliarulo, C.: In Vitro Antibacterial Activity of Pomegranate Juice and Peel Extracts on Cariogenic Bacteria. BioMed Res. Int (2017). https://doi.org/10.1155/2017/2152749
Alexandre, E.M.C., Silva, S., Santos, S.A.O., Silvestre, A.J.D., Duarte, M.F., Saraiva, J.A., Pintado, M.: Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 115, 167–176 (2019). https://doi.org/10.1016/j.foodres.2018.08.044
Hayouni, E.A., Miled, K., Boubaker, S., Bellasfar, Z., Abedrabba, M., Iwaski, H., Oku, H., Matsui, T., Limam, F., Hamdi, M.: Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine 18, 976–984 (2011). https://doi.org/10.1016/j.phymed.2011.02.011
Gullon, B., Pintado, M.E., Pérez-Álvarez, J.A., Viuda-Martos, M.: Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 59, 94–98 (2016). https://doi.org/10.1016/j.foodcont.2015.05.025
Saleem, M., Saeed, M.T.: Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. J. King Saud Univ. - Sci. 32, 805–810 (2020). https://doi.org/10.1016/j.jksus.2019.02.013
Naz, S., Siddiqi, R., Ahmad, S., Rasool, S.A., Sayeed, S.A.: Antibacterial Activity Directed Isolation of Compounds from Punica granatum. J. Food Sci. 72, M341–M345 (2007). https://doi.org/10.1111/j.1750-3841.2007.00533.x
Akiyama, H., Fujii, K., Yamasaki, O., Oono, T., Iwatsuki, K.: Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 48, 487–491 (2001)
Engels, C., Schieber, A., Gänzle, M.G.: Inhibitory spectra and modes of antimicrobial action of gallotannins from mango kernels (Mangifera indica L.). Appl. Environ. Microbiol. 77, 2215–2223 (2011). https://doi.org/10.1128/AEM.02521-10
Widsten, P., Cruz, C.D., Fletcher, G.C., Pajak, M.A., McGhie, T.K.: Tannins and Extracts of Fruit Byproducts: Antibacterial Activity against Foodborne Bacteria and Antioxidant Capacity. J. Agric. Food Chem. 62, 11146–11156 (2014). https://doi.org/10.1021/jf503819t
Fattouch, S., Caboni, P., Coroneo, V., Tuberoso, C., Angioni, A., Dessi, S., Marzouki, N., Cabras, P.: Comparative Analysis of Polyphenolic Profiles and Antioxidant and Antimicrobial Activities of Tunisian Pome Fruit Pulp and Peel Aqueous Acetone Extracts. J. Agric. Food Chem. 56, 1084–1090 (2008). https://doi.org/10.1021/jf072409e
Akinyele, T.A., Okoh, O.O., Akinpelu, D.A., Okoh, A.I.: In-Vitro Antibacterial Properties of Crude Aqueous and n-Hexane Extracts of the Husk of Cocos nucifera. Molecules 16, 2135–2145 (2011). https://doi.org/10.3390/molecules16032135
Pfaller, M.A., Diekema, D.J.: Epidemiology of Invasive Candidiasis: a Persistent Public Health Problem. Clin. Microbiol. Rev. 20, 133–163 (2007). https://doi.org/10.1128/CMR.00029-06
Ishida, K., de Mello, J.C.P., Cortez, D.A.G., Filho, B.P.D., Ueda-Nakamura, T., Nakamura, C.V.: Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 58, 942–949 (2006). https://doi.org/10.1093/jac/dkl377
Mendes de Toledo, C.E., Santos, P.R., Palazzo de Mello, J.C., Dias Filho, B.P., Nakamura, C.V., Ueda-Nakamura, T.: Antifungal Properties of Crude Extracts, Fractions, and Purified Compounds from Bark of Curatella americana L. (Dilleniaceae) against Candida Species. Evid Based Complement Alternat Med (2015). https://doi.org/10.1155/2015/673962
de Souza, C., Vasconcelos, L., Sampaio, M.C.C., Sampaio, F.C., Higino, J.S.: Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Verwendung von Punica granatum als Antimykotikum gegen Candidose in Verbindung mit Zahnprothesen-Stomatitis. Mycoses 46, 192–196 (2003). https://doi.org/10.1046/j.1439-0507.2003.00884.x
Anibal, P.C., Peixoto, I.T.A., Foglio, M.A., Höfling, J.F.: Antifungal activity of the ethanolic extracts of Punica granatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Braz. J. Microbiol. 44, 839–848 (2013). https://doi.org/10.1590/S1517-83822013005000060
Reddy, M., Gupta, S., Jacob, M., Khan, S., Ferreira, D.: Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica granatum L. Planta Med. 73, 461–467 (2007). https://doi.org/10.1055/s-2007-967167
Scalbert, A.: Antimicrobial properties of tannins. Phytochemistry 30, 3875–3883 (1991). https://doi.org/10.1016/0031-9422(91)83426-L
Edwin, H.: Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 59, 205–215 (1996)
Salmon, J.-M.: Interactions between yeast, oxygen and polyphenols during alcoholic fermentations: Practical implications. LWT - Food Sci. Technol. 39, 959–965 (2006). https://doi.org/10.1016/j.lwt.2005.11.005
Devatine, A., Chiciuc, I., Poupot, C., Mietton-Peuchot, M.: Micro-oxygenation of wine in presence of dissolved carbon dioxide. Chem. Eng. Sci. 62, 4579–4588 (2007). https://doi.org/10.1016/j.ces.2007.05.031
Picataggio, S., Rohrer, T., Deanda, K., Lanning, D., Reynolds, R., Mielenz, J., Eirich, L.D.: Metabolic Engineering of Candida Tropicalis for the Production of Long-Chain Dicarboxylic Acids. Nat. Biotechnol. 10, 894–898 (1992). https://doi.org/10.1038/nbt0892-894
Acknowledgements
Authors wish to thank Pr. Abdelmajid DARI for providing advice, scientific help and technical support. Also we are grateful for the scientific support provided by Dr. Said Ezrari to realize the statistical analysis and thankful to all those who directly or indirectly contributed to this research, in particular the kind reviewer and the Editor.
Funding
The financial assistance of Sidi Mohamed Ben Abdellah University (Laboratory of applied chemistry and laboratory of Functional Ecology and Environment) towards this research is hereby acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El moujahed, S., Chahdi, F.O., Rodi, Y.K. et al. The Moroccan Pomegranate: An Underrated Source of Tannins Extracts and Natural Antimicrobials from Juice Processing Byproducts. Waste Biomass Valor 12, 5383–5399 (2021). https://doi.org/10.1007/s12649-021-01413-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01413-1