Abstract
In this study, the extraction conditions extracted maximize amounts of phenolic and bioactive compounds from the fruit extract of Ficus auriculata by using optimized response surface methodology. The antioxidant capacity was evaluated through the assay of radical scavenging ability on DPPH and ABTS as well as reducing power assays on total phenolic content (TPC). For the extraction purpose, the ultrasonic assisted extraction technique was employed. A second-order polynomial model satisfactorily fitted to the experimental findings concerning antioxidant activity (R2 = 0.968, P < 0.0001) and total phenolic content (R2 = 0.961, P < 0.0001), indicating a significant correlation between the experimental and expected value. The highest DPPH radical scavenging activity was achieved 85.20 ± 0.96% at the optimum extraction parameters of 52.5% ethanol (v/v), 40.0 °C temperature, and 22 min extraction time. Alternatively, the highest yield of total phenolic content was found 31.65 ± 0.94 mg GAE/g DF at the optimum extraction conditions. From the LC–ESI–MS profiling of the optimized extract, 18 bioactive compounds were tentatively identified, which may regulate the antioxidant activity of fruits of F. auriculata.
Similar content being viewed by others
Introduction
The human body is vulnerable to reactive oxygen species (ROS). Natural antioxidants are an essential compound for reducing the concentration of these species and prevent various chronic disorders like cancer, rheumatoid arthritis, atherosclerosis, emphysema, cirrhosis, diabetes and others, which cause free radical (˙OH, 1O2, O2˙−) and non-free radical (R–OOH, NO, ONOO−, and H2O2) ROS species1,2. Besides the body’s endogenous antioxidant defence, antioxidants are primarily derived from diet and can promote good health. Numerous synthetic antioxidants are commonly used in different food products, but these products are restricted due to their carcinogenic and other toxic properties3. In addition, there is a demand for natural antioxidants as food preservatives to reduce oxidation and rancidity of foods. Therefore, the attention of natural antioxidants has been raised considerably in the study of certain fruits, vegetables and leaves with high antioxidant contents to boost their consumption. Consequently, an effective extraction technique and the optimization of the extraction conditions are very important for the isolation of antioxidant phenolic compounds. It may enable to obtain natural antioxidants in larger quantities and reduce costs.
Ficus auriculata Lour., a member of the Moraceae family, is a naturally grown plant in lowland tropical rainforests, along streams or on rocks. It is also known as Elephant ear fig or Roxburgh fig4. Its crude extract exhibited antioxidant, antibacterial, antimicrobial, antihyperlipidemic, hepatoprotective activity as well as contain a higher amount of flavonoid content5,6,7. Fruits of F. auriculata are not only valuable for its nutritional value but also contains a higher amount of phenolic compounds as compared to other parts. The previous study found that the leaves, barks, and fruits of F. auriculata exhibited good result with the inhibition of DPPH and ABTS scavenging activity using ultrasonic assisted extraction process8. Moreover, most of the extracts from F. auriculata obtained by ultrasound assisted extraction (UAE) process, showed the highest antioxidant activity, phenolic contents and extraction yields as compared to the maceration process8. In 2014, Hlail and co-workers reported similar phenomena for the fruits to extract, which exhibited higher biological activity compared to leaves extract9.
Numerous extraction techniques have been evolved and used to isolate the bioactive antioxidant compounds from plant sources. Among these techniques, maceration extraction3,10, microwave-assisted extraction11,12 and supercritical fluid extractions13,14 are now used. In the first case, it is time wasting and requires relatively large amounts of solvents. The supercritical fluid extraction process is not economically viable due to the higher cost of the equipment and blockage the systems due to the use of water as the solvent. By considering the concept of “green chemistry”, environment-friendly techniques are required for the determination of antioxidant compounds. Ultrasonic assisted extraction (UAE) is an eco-friendly method, which offers high extraction efficiency, good reproducibility in lower extraction times and requires relatively low solvent, temperature, and energy input. This method can be easily scaled up for industrial applications15,16.
In general, process optimization could be achieved through either statistical or experimental method17,18. The experiential technique involves the study of one-factor-at-a-time which is that all the variables are kept at constant and only one variable changes19. It also increases the experimental run to conduct the research that is laborious, time-consuming and raise the solvent and materials consumption20. So, it is needed to establish the optimum process to recover the highest numbers of bioactive compounds with conserved all the functional parameters. Among the various Response surface methodology (RSM) designs, Central composite design (CCD) is an efficient system which is timesaving and more competent among others. It is very much helpful to develop, improve and optimize extraction conditions of natural antioxidants and plant metabolites21,22. Previously many studies have done to determine the antioxidant activity and TPC of different parts of Ficus auriculata plant. However, to the best of our knowledge, there are very limited studies on extraction of phenolic and bioactive compounds from fruits extract of F. auriculata, in particular no specific study on optimized extraction process.
Therefore, the purpose of this study was to optimize the extraction parameters to extract maximize bioactive and phenolic compounds from the fruits of F. auriculata using UAE and RSM. Finally, the phenolic profile of the most active extract was comprehensively studied by liquid chromatography (LC) coupled to mass spectrometry (MS) via electrospray ionization (ESI).
Materials and methods
Chemicals and reagents
1,1-Diphenyl-2-picrylhydrazyl (DPPH) and 2,2ʹ-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Folin-Ciocalteu reagent was purchased from Merck, Germany. Potassium persulfate, 99.9% pure ethanol, monohydrate gallic acid and anhydrous sodium carbonate were purchased from Friendemann Schmidt (FS) Chemicals, Australia. All the chemicals which were used in this study were in analytical grade. The 18 mΩ deionised water was used to prepare standard materials and extraction.
Sample preparation
The fresh fruit samples of F. auriculata were picked up from the main campus of Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia (Fig. 1) which was cultivated and maintained by the infrastructure and management, UKM. The permission has been taken to collect the fruit sample from the proper authority (Pengarah Prasarana-UKM) and the collection of fruit sample was done by following the institutional, national and international guidelines and legislation. The plant identity was kindly confirmed by Engineer Mohamad Ruzi Bin Abdul Rahman, Herbarium University Kebangsaan Malaysia (HUKM), Faculty of Science and Technology, Universiti Kebangsaan Malaysia. A voucher specimen (Voucher number: ID002/2021) was deposited at the Herbarium University Kebangsaan Malaysia (HUKM), Faculty of Science and Technology, Universiti Kebangsaan Malaysia. The fruits were cleaned properly with distilled water and then dried at 45–50 °C with the help of Septree Food Dehydrator. Finally, all the fruits were powdered using a special grinder (XY-2200B, Shenzhen Yason General Machinery Co., Ltd, Guangdong, China) and stored in an airtight container.
Extraction procedures
The extraction of the fruits of F. auriculata was executed in Thermo-line ultrasonic bath (220 V and 40 kHz) at 35 °C. Two hundred fifty mg of dried and ground powdered sample was transferred into a capped long test-tube (50 mL) and 10 mL of solvent was poured in the sample. Then, the mixture was placed in the ultrasonic bath for sonication. Following extraction, the suspension samples were centrifuged for 15 min at 4000 rpm. Finally, the supernatant liquids were filtered, and the extract thus obtained used directly for the determination of required properties. Figure 2 shows the extraction process of antioxidant active compounds from F. auriculata fruits.
Evaluation of antioxidant characteristics
DPPH assay
The DPPH radical scavenging activity of fruits extract of F. auriculata were measured using previously reported method with some modification23. In brief, 0.1 mM of fresh DPPH was prepared with 70% of aqueous ethanol as the control solution. The 100 μL of different standard Trolox solution (positive control) and the sample were added to 3.9 mL DPPH solution (0.1 mM). Then, the control, and sample absorbance were recorded at 520 nm after incubated 30 min at dark condition and room temperature. The DPPH scavenging activity (percentage of inhibition) was calculated by using the equation below:
where AC is the absorbance of a control solution, AS is the absorbance of standard or sample solution. Each sample and standard were measured in three replications. The absorbance was measured with 756 PC UV–Visible spectrophotometer.
ABTS+ assay
The ABTS radical scavenging assay was calculated based on the method described by Gorinstein et al.24 with little modifications. At first the 7 mM ABTS solution using water was prepared and mixed with 2.45 mM potassium persulfate (K2S2O8) solution with same ratio to get the free radical solution25. In dark condition at room temperature, the mixture was stored for 12–16 h. To carry out each bioassay, the fresh working solution was then made by diluting 1 mL ABTS radical solution with the amount of ethanol needed to achieve an absorbance of 0.700 ± 0.02 units at the wavelength of 745 nm. After that, 100 μL of different standard Trolox solution and extracts sample was added to 3.9 mL of an ABTS+ solution and incubated 6 min at room temperature. Finally, the control and sample absorbance were instantly assessed at 745 nm. Here, Trolox is the positive control and 70% of aqueous ethanol is used as blank. Finally the Eq. (1) was used to calculate the inhibition percentage. The equipment used was described before.
Total phenolic content (TPC) assessment
The TPC of fruits of F. auriculata was assessed using Folin-Ciocalteu (FC) reagent with a little modification26. Prior to use the FC reagent were diluted at 20 times. Then the 100 μL of gallic acid or extract samples were properly added with 3.4 mL of FC reagent and kept for 7 min. A 500 µL of Na2CO3 (20%) was then added to the reaction mixture and incubated at room temperature in a dark place for 2 h. The absorbance was finally determined at 760 nm from a standard gallic acid curve of 31.25 µg/mL to 1000 µg/mL. The outcomes of the TPC were presented as mg gallic acid equivalent (GAE)/g dry fruits (DF). Each experiment was done as triplicate. The equipment used was as for previous assays.
Experimental design
RSM and CCD were used to optimise the three independent variables viz. solvent concentration (X1, %, v/v); extraction temperature (X2, °C) and sonication time (X3, min) at five different levels with responses of two dependent variables such as antioxidant activity (DPPH assay) and TPC (Table 1). The design comprising of 20 experimental runs involving 8 factorial points, 6 axial points, and 6 centre points. The second-order polynomial model in the response surface analysis is demonstrated using the Eq. (2):
where Y is the response function of the independent variables; B0 is a constant, Bi is the linear coefficient, Bij is the second-order interaction, and Bjj is the quadratic coefficients. The variable, Xi is the non-coded independent variables. Here, three independent variables were used and hence n equal to 3. Thus, Eq. (2) is expressed with Eq. (3):
where Y represents the predicted response (antioxidant activity and TPC), and X1, X2 and X3 are independent variables. B0 is a constant and B1, B2 and B3 are linear coefficients. B12, B13 and B23 are cross coefficients and B11, B22 and B33 are quadratic coefficients.
Statistical analysis
Analysis of variance (ANOVA) was used to verify the statistical validity of the response surface quadratic model coefficients and the Design-Expert 6.0.6 (Stat-Ease, Inc., USA) was used to conduct the data analysis. The regression coefficient (R2) along with the F-test, was assessed to test the fit of the polynomial model. The statistical significances for different terms in the polynomial model were evaluated by the estimation of F-value with different probability (P) range such as 0.001, 0.01 or 0.05. P values less than 0.05 and 0.01 indicate that the value is statistically significant and very significant. The % of DPPH inhibition and GAE curve was done using Microsoft Excel 16 (Microsoft Inc., Redmond, USA).
Determination of bioactive compounds via LC–ESI–MS studies
The bioactive phenolic compounds were profiled by LC–MS using the mass analyzer Bruker micrOTOF-Q, Bruker, Germany. A reverse phase C18 column (Phenomenex 250 mm, 5 μm particle size) was used. The eluting system consisted of water acidified with 0.1% formic acid and (1:1, v/v) acetonitrile/methanol acidified with 0.1% formic acid as solvent A and B respectively. The 0.45 μm membrane disk filter was used to filter the mobile phase and degassed by sonication before injection. The parameters which were used to the Elution process are as follows: 5% B, 0–5 min; 5%–10% B, 5–10 min; 10%–50% B, 10–55 min; 50%–95% B, 55–65 min; 5% B, 65–70 min. The 20 μL of solvent was injected with 0.4 mL/min flow rate. The analytical parameters with negative ion mode were performed as follows: source temperature 150 °C, desolvation temperature 350 °C, cone voltage 50 eV, capillary voltage 3 kV, cone gas flow 50 L/h, desolvation gas flow 600 L/h. The ion mass spectra were acquired between m/z 50–1000 and the peaks data were processed using the Bruker Daltonics Data Analysis 3.4 software. By comparing with the retention time of spectra and reported mass spectrum data with the literature on genus Ficus and family Moraceae, the bioactive compounds were identified.
Results and discussion
Impact of solvent on extraction process
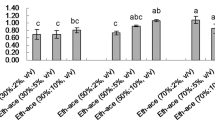
Before using RSM, the impact of solvent type and solvent to solid ratio were studied. Solvent selection is an important tool for the extraction of plant metabolites. Generally, two polar solvents such as methanol (high polarity) and ethanol (medium polarity), are used for the extraction processes when focusing on phenolic compounds. For the extraction purposes, US Food and Drug Administration (UFDA) recommended environment friend and food-grade non-toxic organic solvents and pure methanol is more toxic than the pure ethanol27. In the present study, several of these solvents were used alone or in combination with water. Our results suggested that the efficiency of methanol was higher than single solvent ethanol, ethyl acetate and n-hexane, but lower than the aqueous ethanol (75%) to extract phenolic antioxidant compounds from the fruits of F. auriculata as per the conditions of 10:0.250 (mL/g) solvent to solid ratio, 40 °C temperature and 30 min extraction time (Fig. 3). From our study, the extraction ability of the bioactive phenolic compounds depends on the polarity of the solvent. In this study four solvents were chosen based on the polarity index with different dielectric constant (ε). Methanol is highly polar solvent where ethanol is medium polar and ethyl acetate is low polar solvent. According to the Fig. 3, n-hexane showed very low activity as it is a non-polar solvent with very low dielectric constant (ε = 1.88). Therefore, due to the low toxicity and better extraction ability of aqueous ethanol, it was chosen as the master solvent for each of the next experimental runs for the determination of antioxidant activity and TPC from the fruits of F. auriculata. This agreed with several studies that also found that the combination of water with pure solvent is more effective than solvent alone for extracting phenolic antioxidant compounds28,29. So, the aqueous ethanol was the best solvent to extract polyphenols and the addition of water increased the polarity of the ethanol and the extraction potential in this case.
The influence of solvent to solid ratio on the extraction process from the fruits extract of F. auriculata was also studied with four ratio: 10/0.150, 10/0.250, 10/0.350 and 10/0.450 mL/g, over 75% of solvent, 30 min reaction time and 40 °C temperature. Figure 4 presents the outcomes. The antioxidant activities and TPC increased with the increased amount of solid material in a fixed amount of solvent (10 mL), and it increased up to 0.250 mg of solid. After that, the trend followed a declined efficiency. This is because, the speed of mass transfer depends on the ratio of solvent to solid and increasing ratio enable the distribution of antioxidants into the extraction solvent till maximize the mass transfer. Therefore, the ratio of 10/0.250 (mL/g) was chosen for each of the next experimental runs and to minimize the solvent requirement.
Fitting the RSM models
The results (antioxidant activity and TPC values) of the CCD design are shown in Table 2. Moreover, the response surface quadratic model was used to evaluate the extraction process to maximize the inhibition of DPPH and obtain the highest TPC from the fruit extracts of F. auriculata. Ethanol concentration (X1), temperature (X2) and time of extraction (X3), were used as the independent variables which also commented before. The regression coefficient (R2) was checked to measure the degree of fitness30. When R2 approaches unity, the model can significantly fit well with the predicted values31. The R2 value and ANOVA results of the response surface quadratic models for F. auriculata fruits extracts are compiled in Tables 3 and 4, respectively. In the present study, R2 values for antioxidant activity and TPCwere 0.96, for the quadratic model as well as 0.98 and 0.99 respectively for cubic model, but the design suggested quadratic model and aliased cubic model. The high values of R2 indicate that there is a good relationship between the predicted and experimental values for the models. The degree of precision of a model also can be checked by the coefficient of variations (C.V.). A high value of C.V. indicates the lower reliability of the experiment32. In this study, the C.V. values were 1.17% and 7.47% for antioxidant activity and TPC, respectively, which were low and indicates the executed experiments are highly reliable.
The probability factor (P-value) is another important value to evaluate the significance of independent variables. A lower P-value is highly recommended for significance32. In the present study, the model was significant due to the value of P was less than 0.05. According to Table 4, two linear coefficients such as X1 and X2 and three quadratic term coefficients such as X12, X22 and X32 were significant (P < 0.05) for the response of antioxidant activities. In contrast, two linear coefficients (X1 and X3) and all the quadratic term coefficients (X12, X22 and X32) were significant for the response of TPC. The other terms of coefficients were insignificant due to the P-value was > 0.05. Furthermore, the model F-value for antioxidant activity and TPC were 29.46 and 34.23, respectively (Table 4). The high F-values further confirmed the models significant within the studied range of process conditions. Moreover, the lack of fit for this model also significant (P-value < 0.05). Therefore, all the results proved that the model fitness was adequate and both models were fully applicable. Figure 5 represents the Predicted vs Actual values for Antioxidant activity and TPC. The perfect fit line Predicted = Actual values with a high degree of correlation with best fit line equation y = mx + c indicates the best accuracy of the current model.
Impact of extraction parameters on DPPH assay
The effects of solvent, extraction temperature and sonication time on the DPPH assay of fruit extracts of F. auriculata as well as their interactions are shown in Table 2 and Fig. 6. Equation (4) displays the correlation between independent variables for the DPPH radical scavenging activity of F. auriculata fruits extracts.
where Y1 represents the DPPH radical scavenging activity in fruit extracts of F. auriculata. X1, X2, and X3 represents the solvent concentration (%), temperature (°C) and time (min), respectively.
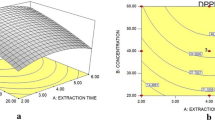
The DPPH assay was selected since it is a broadly used and reliable antioxidant determination method compared to other assays33. In this process, DPPH solution reduced to non-radical DPPH-H in the presence of hydrogen-donating antioxidants. The antioxidant compound containing crude fruit extract of F. auriculata fruits reduced the stable purple colour to yellow-coloured diphenylpicryl-hydrazine. The experimental values of DPPH assay with various extraction conditions are shown in Table 2. The variables studied here, the concentration of ethanol, sonication temperature and sonication time, showed the effects on the antioxidant activity of fruit extract of this plant. At constant sonication time (20 min), the ethanol concentration and temperature effect on DPPH inhibition of F. auriculata fruit extract seemed as light-saddled shapes (Fig. 6a). The ethanol concentration (P < 0.0001) and the temperature were the main significant extraction parameters for antioxidant activity. The effects of sonication time were not statistically significant (P > 0.05), but their quadratic terms were significant as commented before. The DPPH inhibition increases with the growth of ethanol concentration from 7.95 to 52.50%, and thereafter it followed the declining trend at the higher solvent concentration of 92.05%.
The similar trend also found for ultrasound irradiation time for this study (Fig. 6b). The DPPH radical scavenging activities increased from 3 to 22 min and followed by a decreasing trend at longer ultrasound irradiation time. Maximum 84.03% of inhibition was obtained at 22 min. When the ethanol concentration and sonication time were kept constant, the antioxidant activity of the extracts enlarged to a value with the temperature and then started to decrease (Fig. 6c). These studies evidently exhibit that the change of ethanol concentration and temperature, change the activity of DPPH positively in the medium region, and thereafter follow the negative trend for any range of extraction time.
Concerning other studies, similar DPPH radical scavenging antioxidant response plots were also reported by Shahinuzzaman et al. for F. carica latex34, Yang et al. earlier for longan fruit polysaccharides33, Ilaiyaraja et al. for fruit extract of Feronia limonia35 and Liyana-Parthirana and Shahidi for wheat extracts17. In ultrasound assisted extraction, the DPPH radical scavenging activities of the fruit extract of F. auriculata were higher than those of Shirzad and co-worker reported leaves of Olea europaea (78.98%)36, Li et al. reported the antioxidant activity of leaves extract of P. frutescens (73.66%)21; Ilaiyaraja and co-workers reported fruit extract of F. limonia (83.8%)35, Tabaraki and Nateghi reported rice bran (52.83%)18. Previously, the antioxidant activity with DPPH assay of Actinidia chinensis fruit seed extract was studied to optimize the extraction parameters. Using the optimum extraction conditions, it obtained 63.25% antioxidant activity with DPPH assay37. However, the maximum antioxidant activity value obtained for F. auriculata was lower than that reported for olive leaves (95.56%)38.
Impact of extraction parameters on TPC
The effects of the extraction parameters, on the TPC of fruit extracts of F. auriculata under UAE is presents in Table 2. The effect of ethanol concentration and sonication time was decidedly significant as well as the effects of temperature was not statistically significant on the extraction of phenolic compounds. However, multiple regression analysis indicated that the quadratic terms (X12, X22 and X32) were highly significant (p < 0.0001) for the extraction of TPC and is revealed in Table 3, as for the antioxidant activity. So, consistent with the experimental values, the model made the second-order polynomial equations to exhibit the correlation between ethanol concentration, temperature, and time for the TPC (Y2), and is represented in Eq. (5):
A 3D response surface plots were established to obtain the optimum extraction parameters for TPC based on Eq. (5). When sonication time was kept constant (20 min), the effect of solvent and temperature on TPC seemed as a curved shape (Fig. 7a). The TPCs linearly increases with uplifting the ethanol concentration until it reaches a highest limit and then reduced. The highest recovery of phenolics was gained at a solvent concentration between 45 and 55% and temperature between 38 and 43 °C. TPC gradually mounted up and attained a maximum content (~ 33.88 mg TE/g DF) and followed by a sharp decrease afterwards. In this study, the TPC was meaningfully affected by the varying concentration of ethanol and the extraction of phenolic compounds was higher at 52.5% of ethanol thereafter it decreased at the higher concentration of ethanol (75–92.04%). These results are interesting to minimize the global process cost due to the use of ethanol as a solvent.
Response surface plots showing effects of ethanol concentration, temperature, and sonication time on total phenolic contents of the extracts from fruits of F. auriculata. (a) The constant ultrasonic time (20 min), (b) the constant extraction temperature (40 °C), and (c) the constant ethanol concentration (50%).
At constant temperature (40 °C), the relationship of sonication time and concentration of ethanol on TPC is exposed in Fig. 7b. The concentration of ethanol revealed a prominent impact on TPC in a quadratic manner (Table 3). The TPC increases with increasing the ethanol concentration up to 52.5% and additional concentration of ethanol reduced the TPC, probably for the polarity change of the solvent mix. To enhance the yield of phenolic compounds, temperature plays a vital role to soft the plant tissues, increase the solubility and dispersion coefficient of the constituents35. In this case, lower recovery of TPC obtained at the higher temperature (65 °C), and 52.5% of ethanol agrees well. The results found from this study are more favourable compared to previous studies which presented equivalent or higher for the fruit extract of F. limonia35, leaves extract of P. frutescens21, rice bran18, extracts of grape cane39, peels extract of Mangifera pajang40 etc. At constant ethanol concentration, the effect of temperature and sonication time on the yield of TPC is shown in Fig. 7c. TPC of fruit extracts of F. auriculata increased sharply with increasing temperature up to 40 °C and thereafter decreased slightly. This phenomenon observed in our study at moderate temperature due to it could soften the plant tissue, weaken the integrity of the cell wall, hydrolyze the bonds between phenol-polysaccharide or phenol–protein and enrich the solubility of phenolics, thus more phenolic compounds would pass to the extraction solvent41.
Validation of the optimal extraction conditions
The optimum operating conditions were performed in DOE software based on each experimental run and combination of the two responses. The goal of this study was to obtain the highest antioxidant activity and yield of total phenolic content from the fruit extracts of F. auriculata within the range of extraction parameters. To optimise the extraction parameters of antioxidant activity, an ethanol concentration of 52.5% (v/v), the temperature of 40 °C, and ultrasound irradiation time of 22 min were chosen. The highest TPC also found at the same optimum extraction parameters. These optimum conditions gave the highest response value of 84.03% for DPPH assay and 33.88 mg GAE/g DF for TPC, which was forecasted from the model (Table 5).
The validation of the model was also checked at the predicted conditions. The optimal conditions were also tested by using one more radical scavenging assay, i.e. ABTS assay. The outcomes of the experiments showed the following values: 85.20 ± 0.96% for DPPH, 99.12 ± 0.85% for ABTS and 33.25 ± 0.94 mg GAE/g DF for the TPC, which were reliable with the predictive value. The strong relationship between the predicted and experimental values confirmed that the model is correct and consistent in finding the optimal conditions for antioxidants activity and TPC from the fruit extracts of F. auriculata. Therefore, the optimized condition of the proposed protocol can be the easy and time saving way with minimal use of solvent and highest antioxidant activity to extract the bioactive compound from fruits. However, the future trends of this work to keep working with the obtained data is to use other biological assays for antioxidant and TPC as well as the study of sonication power for extraction, harvesting process, season of fruit collection and fertilization.
Characterization of bioactive compounds at optimized extract using LC–ESI–MS
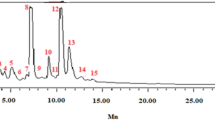
The characterization of phenolic compounds was performed by LC–ESI–MS in the negative ionization mode. For that, the most active extract was studied in depth (Fig. 8): fruit of F. auriculata extracted through the ultrasonication extraction at the optimised extraction process. The retention time (RT), experimental m/z of negative molecular ions ([M−H]−), in-source fragments42, and the proposed compounds are shown in Table 6. The tentative compounds were compared with the reported literature and databases. A total of 18 bioactive compounds were characterized in F. auriculata for first time so far as we know, but few of them were reported in other species. In this way, the preliminary structure of derivatives of caffeoylquinic acid (compounds 2–4), linolenic acid (compound 16) were proposed on the basis of their m/z and fragments. For example, the ion m/z 353 may indicate the presence of a caffeoylquinic moiety in compounds 2, 3 and 4. The unique hydroxycinnamic acid found in the extract was caffeoylquinic acid (compound 2–4), whose occurrence were previously reported in F. carica fruits43,44. Flavanols were represented by A-type trimer (compounds 8) (m/z 863) as also described Vallejo and co-workers (2012) in F. carica fruits44. Their fragmentation patterns agreed with previous studies by observing the monomer unit (m/z 289), dimer (m/z 577), a fragment ion derived from a retro-Diels–Alder fission at m/z 425 and its subsequent loss of water (m/z 407) depending on the compound45,46. Isoflavones consisted of three compounds such as trihydroxy-octadecadienoic acid, trihydroxy octadecanoic acid and hydroxy-octadecatrienoic acid (compounds 10, 11, 13). Most of them have been reported in several Ficus species, including F. carica, F. tikoua, and F. mucuso43,46,47.
These results are highly promising and further studies should be addressed to purify the novel molecules and elucidate their stereochemistry by nuclear magnetic resonance and quantify the compounds using HPLC–DAD-MS, since LC–ESI–MS is limited in this sense.
Conclusions
UAE is an environmentally friendly, simple, and economical extraction process for the extraction of antioxidants from the fruits of F. auriculata. The correlation coefficient of this model was high and suggested that a second-order polynomial model should be used. The highest DPPH and ABTS radical scavenging assay as well as reducing power assays were obtained with the optimized extraction conditions. The predicted and experimental data were almost similar. The profiling of phenolic compounds of the optimized extract by LC–ESI–MS revealed the existence of phenolic acids, flavanols, and isoflavones. This study revealed that the fruits of F. auriculata can be a good natural source of antioxidants and phenolic contents. The results of this study supply valuable information to the food and pharmaceutical industries for the extraction of bioactive compounds from the optimized fruit extract. This may also secure the supplementation of bioactive components in a variety of food products aimed at combating oxidative-stress related medical issues through cell metabolism.
References
Huang, D., Ou, B. & Prior, R. L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53, 1841–1856 (2005).
Yoshihara, D., Fujiwara, N. & Suzuki, K. Antioxidants: Benefits and risks for long-term health. Maturitas 67, 103–107. https://doi.org/10.1016/j.maturitas.2010.05.001 (2010).
Chatha, S. A. S., Hussain, A. I., Bajwa, J. U. R. & Sagir, M. Antioxidant activity of different solvent extracts of rice bran at accelerated storage of sunflower oil. J. Food Lipids 13(4), 424–433. https://doi.org/10.1111/j.1745-4522.2006.00068.x (2006).
Huxel, A. (ed.) The New RHS Dictionary of Gardening (MacMillan Press, 1992).
Thingbaijam, R., Dutta, B. K. & Paul, S. B. In vitro antioxidant capacity, estimation of total phenolic and flavonoid content of Ficus auriculata lour. Int. J. Pharm. Pharm. Sci. 4(4), 518–521 (2012).
Gaire, B. P. et al. Phytochemical screening and analysis of antibacterial and antioxidant activity of Ficus auriculata (Lour.) stem bark. Pharmacogn. J. 3, 49–55 (2011).
El-Fishawy, A., Zayed, R. & Afifi, S. Phytochemical and pharmacological studies of Ficus auriculata Lour. J. Nat. Prod. 4, 184–195 (2011).
Akhtar, P., Yaakob, Z., Ahmed, Y. & Shahinuzzaman, M. Ficus species good sources of natural antioxidant drugs. Turk J. Pharm. Sci. 2 (2019).
Hlail, S. H., Ahmad, W. J. W. & Abdullah, A. Variations in antioxidant content in leaves and fruits of Ficus fistulosa. AIP Conf. Proc. 1614, 619–623. https://doi.org/10.1063/1.4895274 (2014).
Japón-Luján, R., Luque-Rodríguez, J. M. & Luque de Castro, M. D. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 1108(1), 76–82. https://doi.org/10.1016/j.chroma.2005.12.106 (2006).
Dahmoune, F., Nayak, B., Moussi, K., Remini, H. & Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 166, 585–595. https://doi.org/10.1016/j.foodchem.2014.06.066 (2015).
Ranic, M. et al. Optimization of microwave-assisted extraction of natural antioxidants from spent espresso coffee grounds by response surface methodology. J. Clean Prod. 80, 69–79. https://doi.org/10.1016/j.jclepro.2014.05.060 (2014).
Ghafoor, K., Park, J. & Choi, Y. H. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 11(3), 485–490. https://doi.org/10.1016/j.ifset.2010.01.013 (2010).
Maran, J. P., Manikandan, S., Priya, B. & Gurumoorthi, P. Box-Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L.) leaves. J. Food Sci. Technol. 52(1), 92–104. https://doi.org/10.1007/s13197-013-0985-z (2015).
Chemat, F., Tomao, V. & Virot, M. Ultrasound-assisted extraction in food analysis. In Handbook of Food Analysis Instruments, 85–103 (2008).
Akhtar, P., Yaakob, Z., Ahmed, Y., Shahinuzzaman, M. & Mar Contreras, M. Potential of leaves of eighteen cultivars of Ficus carica as antioxidants and profiling of phenolic compounds as an active molecules. Iran. J. Pharm. Sci. 15(2), 41–60 (2019).
Liyana-Pathirana, C. & Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 93(1), 47–56. https://doi.org/10.1016/j.foodchem.2004.08.050 (2005).
Tabaraki, R. & Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrasonics Sonochem. 18(6), 1279–1286. https://doi.org/10.1016/j.ultsonch.2011.05.004 (2011).
Baş, D. & Boyacı, İH. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 78(3), 836–845. https://doi.org/10.1016/j.jfoodeng.2005.11.024 (2007).
Bezerra, M. A., Santelli, R. E., Oliveira, E. P., Villar, L. S. & Escaleira, L. A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5), 965–977. https://doi.org/10.1016/j.talanta.2008.05.019 (2008).
Li, H. Z. et al. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and rosmarinic acid from F. perilla leaves using response surface methodology. Food Sci. Technol. 36(4), 686–693 (2016).
Chen, M., Zhao, Y. & Yu, S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 172, 543–550. https://doi.org/10.1016/j.foodchem.2014.09.110 (2015).
Mohammedi, Z. & Atik, F. Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) Karst. Int. J. Pharma Bio Sci. 2, 609–615 (2011).
Gorinstein, S. et al. A comparative study of phenolic compounds and antioxidant and antiproliferative activities in frequently consumed raw vegetables. Eur. Food Res. Technol. 228(6), 903–911. https://doi.org/10.1007/s00217-008-1003-y (2009).
Teixeira, D. M., Canelas, V. C., do Canto, A. M., Teixeira, J. & Dias, C. B. HPLC-DAD quantification of phenolic compounds contributing to the antioxidant activity of Maclura pomifera, Ficus carica and Ficus elastica extracts. Anal. Lett. 42(18), 2986–3003 (2009).
Wang, M., Liu, J. R., Gao, J. M., Parry, J. W. & Wei, Y. M. Antioxidant activity of Tartary buckwheat bran extract and its effect on the lipid profile of hyperlipidemic rats. J. Agric. Food Chem. 57(11), 5106–5112 (2009).
Bartnick, D., Mohler, C. & Houlihan, M. Methods for the production of food grade extracts. U.S. Patent Application 10/972,751; 20060088627, April 27, Sensient Flavors Inc, 2006).
Wang, L., Wang, Z. & Li, X. Optimization of ultrasonic-assisted extraction of phenolic antioxidants from Malus baccata (Linn.) Borkh. using response surface methodology. J. Sep. Sci. 36(9–10), 1652–1658. https://doi.org/10.1002/jssc.201300062 (2013).
Shahinuzzaman, M. et al. In vitro antioxidant activity of Ficus carica L. latex from 18 different cultivars. Sci. rep. 10(1), 1–14 (2020).
Nath, A. & Chattopadhyay, P. K. Optimization of oven toasting for improving crispness and other quality attributes of ready to eat potato-soy snack using response surface methodology. J. Food Eng. 80(4), 1282–1292. https://doi.org/10.1016/j.jfoodeng.2006.09.023 (2007).
Sin, H. N., Yusof, S., Sheikh Abdul Hamid, N. & Rahman, R. A. Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Food Eng. 73(4), 313–319. https://doi.org/10.1016/j.jfoodeng.2005.01.031 (2006).
Yin, X., You, Q. & Jiang, Z. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 86(3), 1358–1364. https://doi.org/10.1016/j.carbpol.2011.06.053 (2011).
Yang, B., Zhao, M., Shi, J., Yang, N. & Jiang, Y. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chem. 106(2), 685–690. https://doi.org/10.1016/j.foodchem.2007.06.031 (2008).
Shahinuzzaman, M. et al. Optimization of extraction parameters for antioxidant and total phenolic content of Ficus carica L. latex from White Genoa cultivar. Asian J. Chem. 31, 1859–1865 (2019).
Ilaiyaraja, N., Likhith, K. R., Sharath Babu, G. R. & Khanum, F. Optimisation of extraction of bioactive compounds from Feronia limonia (wood apple) fruit using response surface methodology (RSM). Food Chem. 173, 348–354. https://doi.org/10.1016/j.foodchem.2014.10.035 (2015).
Shirzad, H., Niknam, V., Taheri, M. & Ebrahimzadeh, H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: A nutraceutical study using RSM and LC–ESI–DAD–MS. J. Food Sci. Technol. 54(8), 2361–2371. https://doi.org/10.1007/s13197-017-2676-7 (2017).
Deng, J. et al. Extraction optimization of polyphenols from waste kiwi fruit seeds (Actinidia chinensis Planch.) and evaluation of its antioxidant and anti-inflammatory properties. Molecules 21(7), 832. https://doi.org/10.3390/molecules21070832 (2016).
Şahin, S. & Şamlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 20(1), 595–602. https://doi.org/10.1016/j.ultsonch.2012.07.029 (2013).
Karacabey, E. & Mazza, G. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem. 119(1), 343–348. https://doi.org/10.1016/j.foodchem.2009.06.029 (2010).
Prasad, K. N. et al. Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm. peels. Food Chem. 128(4), 1121–1127. https://doi.org/10.1016/j.foodchem.2011.03.105 (2011).
Spigno, G., Tramelli, L. & De Faveri, D. M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 81(1), 200–208. https://doi.org/10.1016/j.jfoodeng.2006.10.021 (2007).
Contreras, M. D. M., Arráez-Román, D., Fernández-Gutiérrez, A. & Segura-Carretero, A. Nano-liquid chromatography coupled to time-of-flight mass spectrometry for phenolic profiling: A case study in cranberry syrups. Talanta 132, 929–938. https://doi.org/10.1016/j.talanta.2014.10.049 (2015).
Ammar, S., Contreras, M. D. M., Belguith-Hadrich, O., Bouaziz, M. & Segura-Carretero, A. New insights into the qualitative phenolic profile of Ficus carica L. fruits and leaves from Tunisia using ultra-high-performance liquid chromatography coupled to quadrupole-time-of-flight mass spectrometry and their antioxidant activity. RSC Adv. 5(26), 20035–20050. https://doi.org/10.1039/C4RA16746E (2015).
Vallejo, F., Marín, J. & Tomás-Barberán, F. A. Phenolic compound content of fresh and dried figs (Ficus carica L.). Food Chem. 130(3), 485–492 (2012).
Cádiz-Gurrea, M. L. et al. Isolation, comprehensive characterization and antioxidant activities of Theobroma cacao extract. J. Funct. Foods 10, 485–498. https://doi.org/10.1016/j.jff.2014.07.016 (2014).
Wei, S., Wu, W. & Ji, Z. New antifungal pyranoisoflavone from Ficus tikoua Bur. Int. J. Mol. Sci. 13(6), 7375 (2012).
Bankeu, J. J. K. et al. Isoflavone dimers and other bioactive constituents from the Figs of Ficus mucuso. J. Nat. Prod. 74(6), 1370–1378. https://doi.org/10.1021/np100896w (2011).
Acknowledgements
The authors would like to thank Universiti Kebangsaan Malaysia (UKM) for its financial support to this research work under the grant DIP-2018-007. Authors also acknowledge the BOLD publication support (J510050002-BOLDRefresh2025) from the iRMC of Universiti Tenaga Nasional (UNITEN).
Author information
Authors and Affiliations
Contributions
M.S. and P.A. wrote the main manuscript text, Y.A. prepared Figs. 4, 5 and responsible for the conceptualization of the work, N.A., F.H.A., M.A., and H.M. reviewed and edited the manuscript. M.A. funded for the work. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shahinuzzaman, M., Akhtar, P., Amin, N. et al. New insights of phenolic compounds from optimized fruit extract of Ficus auriculata. Sci Rep 11, 12503 (2021). https://doi.org/10.1038/s41598-021-91913-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91913-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.