Abstract
The occurrence of fish diseases contributes to massive economic loss to the aquaculture industry. Edwardsiella tarda, a known pathogenic organism in Oreochromis mossambicus, infects several freshwater fishes. The present study attempts to find an effective treatment against E. tarda infection using natural plant-based bioproducts. Tamarindus indica seed extract was used to study its antibacterial activity against E. tarda. The study revealed that the petroleum ether extract and ethanolic extracts were effective in controlling the infection. It was found to be effective in both in vitro and in vivo. A maximum zone of inhibition, i.e., 9 and 24 mm, was recorded when 40μL of the petroleum ether extract and 40 μL of the ethanolic extract were loaded separately in the well against the test organism. Results showed that the petroleum ether and ethanol extract of T. indica seed were effective in treating the E. tarda infection.
Similar content being viewed by others
Introduction
Edwardsiella tarda is a gram-negative, facultative anaerobic, motile, rod-shaped bacterium which belongs to the family Enterobacteriaceae. It causes a disease Edwardsiellosis which has been reported globally in several important fish species such as Japanese eel (Anguilla japonica), red sea bream (Pagrus major), yellowtail (Seriola quinqueradiata), etc. (Ewing et al. 1965; Hoshina 1962; Yasunaga et al. 1982; Meyer and Bullock 1973 ; Nougayrede et al. 1994). Although there has been much research and many studies on Edwardsiellosis for many years, the major mechanism by which pathogenesis is caused is still poorly understood. It is an intracellular pathogen that can infect various cell types (H.W. et al. 1980) in a broad range of hosts including birds, fishes, reptiles, amphibians, and even humans (Thomas et al. 2013a; Perez and Lewis 2006). The infection by E. tarda causes a large number of mortalities in fishes and significant commercial losses in the natural environment and also in the fish farming worldwide (Plumb et al. 1993). Xu and Zhang (2014) reported that the major characteristics of Edwardsiellosis include hernia, exophthalmia, and severe lesions of organs and eventual death. The onset and severity of E. tarda infections in fishes is primarily due to environmental stresses like sudden change of water temperature, malnutrition, overcrowding, pH, and variations in dissolved oxygen (Plumb et al. 1993).
Chemotherapy is a commonly used method to control or protect fishes from Edwardsiellosis. Presently oxytetracycline, norfloxacin, ciprofloxacin, gentamycin, chloramphenicol, cefazolin, and aztreonam are some of the antibiotics which are used to control the infection in spite of disadvantages like development of disease-resistant strains, due to indiscriminate use by the farmers (Mohanty and Sahoo 2007). There is therefore a need for the development of some alternative approaches to deal with the control of infection in these organisms.
Nowadays, natural and plant-based extracts are being extensively used to protect fishes from different infections and diseases. They were found to have antimicrobial, antioxidant, and immunostimulatory activity against many pathogenic bacteria. In addition, many of these plant-based extracts have been used to control the diseases such as streptococcosis (Abutbul et al. 2004; Rattanachaikunsopon and Phumkhachorn 2007; Rattanachaikunsopon and Phumkhachorn 2009) and motile Aeromonas septicemia in fishes (Pachanawan et al. 2008).
Materials and methods
Collection and maintenance of fishes
Healthy disease-free fishes, Oreochromis mossambicus without any clinical signs weighing approximately 10 gm ± 2, were obtained from a fish farm in Vellore district, Tamil Nadu, India. The fish were determined to be disease-free since they did not show symptoms of any disease. The fishes were transported to the laboratory in live condition with continuous aeration using a battery-powered aerator and maintained in large glass tanks with continuous aeration at room temperature (27–30°C). The animals were maintained in static water. Commercial fish feed was given twice a day.
Collection of plant material
The seeds of tamarind (Tamarindus indica) were obtained from fresh tamarind which was bought from the market. The seeds were then shade dried and crushed in a mixer grinder, after which they were stored properly in an airtight container.
Preparation of plant extract
The seeds were ground in a mixer grinder. After grinding, an extract was prepared using ethanol, petroleum ether, and dichloromethane as solvents by adopting the method followed by Montoro et al. (2006) and Chandrasekhar Reddy et al. (2011). The ground powder of the seed (5g) was dissolved in the respective solvents (30ml) and then kept in a shaker for 24–48 h after which it was filtered. The filtrate was then dried in a hot air oven at 70–80°C. The dried extract was then scraped and stored in the refrigerator. This was used for in vitro and in vivo studies (Logambal et al. 2000).
Bacterial culture
The culture, E. tarda is a fish pathogen (Jaruratanasirikul and Kalnauwakul 1991; Janda et al. 1991; Janda and Abbott 1993). E. tarda was purchased from Microbial Type Culture Collection (MTCC 2400), Chandigarh, India. The bacterial culture was confirmed by the biochemical reaction scheme provided by Berges Manual of Systemic Bacteriology (1984). It was cultured on nutrient agar and used for pathogenicity experiments.
Experimental pathogenicity in healthy fishes by bath exposure and intramuscular injection
Five healthy fishes per tank were reared in glass tanks of 50-l capacity and were filled with freshwater. Aeration was provided continuously. The tanks were covered with fine nylon net to prevent the entry of small insects. Sterile condition was maintained during the course of the experiment. The fishes were fed with commercial fish feed. The bacterial culture was grown on nutrient broth at 37°C and centrifuged at 6500 rpm for 10 min. The pellet was collected and washed with 0.9% saline. It was suspended in 0.5 ml of 0.9% saline. Different concentrations (101, 102, 103, 104, 105 CFU/ml) were prepared and used for injection. For experimental infection, various concentrations (101 to 105) of bacterial cell suspension were added to the water (H.W. et al. 1980; Thanigaivel et al. 2015). The animals were monitored for a period of 120 h. The bacterial numbers were determined by hemocytometer slide. The experiments were conducted in triplicates. Experimental pathogenicity in healthy fish was performed by intramuscular injection following the method of Thomas et al. (2014) and Thomas et al. (2013b). Pathogenicity was induced by intramuscular injection of 20 μl (105) of bacterial suspension of the respective concentration in to the fishes. Control fishes were injected with sterile phosphate-buffered saline (PBS) buffer. The experiment was conducted in triplicates. Symptoms and mortality of the fishes were checked twice a day. Dead fishes were removed and preserved for further studies The re-isolation of the bacteria was performed from the experimentally infected fishes and further confirmed by the biochemical characteristics. Re-isolation of the bacterium from infected fish was done to satisfy Koch’s postulates.
Toxicity assessment
ROS (reactive oxygen species) assay
To determine the effect of ROS production by phagocytes, five fish per group were maintained. Three groups of fishes were maintained. Group 1 was fishes injected with the bacteria, group 2 was fishes treated with extract, and group 3 served as a control. ROS production was measured by using peripheral blood leukocytes (PBL) at regular intervals. The intracellular respiratory burst activity was performed by the nitroblue tetrazolium (NBT) reduction assay by following the method of Secombes (1990) with minor modifications. PBL were incubated with 25 μl of NBT containing 175-μl culture medium. It was kept for 2 h at 28°C. The supernatants were removed and the cells were fixed with 100% [v/v] methanol for 5 min. Each well was washed twice with 125 mL of 70% [v/v] methanol. The cells were allowed to dry. The reduced NBT (in the form of formazan) was dissolved using 125 μl of 2N potassium hydroxide (KOH) and 150-μl dimethyl sulfoxide (DMSO) in each well. OD value was recorded spectrophotometrically at 650 nm (Šípová et al. 2011; Wang and James 1999)
Treatment of fishes with the extract
Three experimental groups were set up for the experiment. Group 1 fishes served as control throughout the experiment. They were fed with commercial fish feed and were not treated with the extract. For treatment group (group 2), 50 mg/l of prepared dried ethanolic extract was added to the experimental tank. Group 3 fishes were not treated with the extract. In groups 2 and 3, the fishes were treated with the bacteria at concentration 103 which was the highest concentration noted in the pathogenicity experiments (Ayalew and Fufa 2018). The relative percentage of survival (RPS) was studied by Kubilay et al. (2008) and Amend (1981).
Identification of phytocomponents in Tamarindus indica by GC-MS (gas chromatography-mass spectrometry)
The different solvent extracts (ethanol, petroleum ether, dichloromethane) of the seed were tested for the presence of chemical components, secondary metabolites, and natural bioactive products using GC-MS. The solvent extract which was found to be effective was used to identify the compounds present in the extract. A small amount of sample was injected using Hamilton syringe to the GC-MS manually in split mode (Thomas et al. 2014; Thanigaivel et al. 2014).
Biochemical parameters
The biochemical parameters of infected, control, and treated organs were determined. Total protein, free amino acids, glucose, total carbohydrate, cholesterol, and free fatty acids were determined in experimental and control fishes as mentioned below.
Total protein estimation
Total protein content of the sample was estimated spectrophotometrically at 640 nm according to the standard method of Lowry et al. (1951).
Total carbohydrate
The concentration of total carbohydrate was determined by the method of Hedge and Hofreiter (1951).
Glucose
Glucose content was determined by the glucose oxidase method of Malik and Singh et al. (1980).
Estimation of triglycerides
The method followed by Lowell B. and Ralph T. (1973) was adopted to estimate the triglycerides content. Neutral lipids were extracted from the tissue lipid aliquot with isopropanol in the presence of alumina. Glycerol was released by saponification and oxidized to form formaldehyde. The latter was condensed with ammonia and acetyl acetone and absorbance of the reaction mixture was measured at 405 nm. For this, 0.1 ml of lipid extract and 3.0 ml of isopropanol were added and mixed well. This was followed by the addition of 400 mg of activated alumina. The tubes were shaken well in a vortex mixer and the proteins were centrifuged off. In 2.0 ml of the supernatant obtained from the sample, 0.6 ml of saponifying reagent was added and placed in a water bath at 60–70°C for 15 min. Standard tripalmitin (20–100 μg) solution was also saponified in a similar manner. The tubes were cooled, and 1.0 ml of sodium metaperoxidate solution and 0.5 ml of acetyl acetone were added and mixed well. The tubes were then allowed to cool to room temperature and the color developed was read at 405 nm against the reagent blank. The amount of triglycerides was expressed as milligram per gram tissue. Serum triglycerides were expressed as milligram per deciliter.
Assay of glutathione S-transferase
The modified protocol of William et al. (1974) was performed to measure the glutathione S-transferase activity. The reaction mixture containing 1.0 ml of buffer, 0.1 ml of 1-chloro-2,4-dinitrobenzene (CDNB), and 0.1 ml of fish tissue homogenate was made up to 2.5 ml by the addition of water. This reaction mixture was pre-incubated at 37°C for 5 min. Of GSH solution, 0.1 ml was then added. The absorbance was measured at 340 nm for 3 min at 30-s intervals. The enzyme activity was expressed as mMoles of CDNB conjugate formed/min/mg protein.
Super oxide dismutase
The activity of SOD was measured by following the slight modified method of Beauchamp and Fridovich (1995) using nitroblue tetrazolium (NBT) in the presence of riboflavin. Tissue sample preparation for the SOD activity was done as follows: 100 mg of tissues were homogenized in a mechanical homogenizer containing 0.5 ml of phosphate buffer (50 mM, pH 7.8). The homogenate was then centrifuged at 5724 × g for 5 min at 4°C, in a C24 Remi cooling centrifuge. The supernatant was collected and heated for 5 min at 65°C. The supernatant collected after heating was stored at –20°C until use. The obtained protein samples were then maintained in ice in order to avoid the denaturation of protein.
Assay of catalase
The method of Sinha (1972), was adopted for the assay of catalase activity. The tissue homogenate was ground with 0.01 molar PBS buffer at pH 7. In the tissue homogenate, 0.4 ml of hydrogen peroxide was added and the reaction was arrested after 30 and 60 s by addition of 2.0 ml of dichromate acetic acid. All the reaction tubes were boiled in a water bath for 10 min, then cooled, and absorbance recorded at 620 nm. The activity was expressed as units/min/mg/protein.
Estimation of lipid peroxidation
The technique described by Aotsuka et al. (1979) was followed to estimate the lipid peroxide content by thiobarbituric acid (TBA) reaction. To the sample (0.1 ml), 1.5-ml acetic acid, 1.5-ml TBA reagent, and 0.2-ml sodium dodecyl sulfate (SDS) reagent were added and made up to 5ml using distilled water. Standards and blank were also treated similarly. The tubes were incubated for 1 h in boiling water and then centrifuged at 10,000 rpm for 10 min. The pink color developed was measured at 532 nm. The results were recorded as ng of TBA reactive substances/milligram protein.
Immunological parameters
Total erythrocyte and leukocyte counts
Determination of immunological parameters in the blood samples collected from the control, infected, and treated fishes was done by measuring red blood cells count (RBC) count and white blood cells (WBC) count by the standard procedure. This was carried out by diluting with the respective blood fluids, namely RBC and WBC diluting fluid. It was performed by adding 20 μL of blood samples with 3980 μl of corresponding diluting fluid in an aseptic test tube. Cell counts were performed using a Neubauer’s counting chamber. Diluting fluids were prepared as follows.
WBC diluting fluid
Of glacial acetic acid, 200 μl was mixed with 10ml of distilled water.
RBC diluting fluid
Three grams sodium citrate was mixed with 1 ml of formalin. The final content was made up to 100ml.
Results and discussion
Experimental pathogenicity
The pathogenicity of E. tarda to O. mossambicus was assessed by bath exposure method and intramuscular method. Highest concentration of 101 and 102 viable cells of E. tarda caused 80 and 60% mortality within 72 and 48 h of post-inoculation respectively in the bath exposure method. The cells at lower concentration 104 and 105 of cells caused 60 and 50% of mortality, respectively, within 48 and 72 h of post-inoculation. In the intramuscular injection method, the highest concentration, 102 and 103 live cells of E .tarda, caused 70 and 60% mortality within 120 and 96 h of post-inoculation, respectively. Of bacteria, 104 and 105 viable cells caused 30 and 25% mortality after 120 and 96 h of post-injection, respectively. From the pathogenicity study, it may be concluded that the site of entry of pathogens is by wounds or lesions on the surface or some mechanical injury as reported by Thomas et al. (2014). They also reported that the mortality data in the pathogenicity experiments depends on the dosage and period of exposure to the infectious agent (Thomas et al. 2014).
Toxicity assessment
The reactive oxygen species (ROS) production suggests that there was significant increase in the ROS level in the infected fishes when compared to the treated and normal fishes. Reactive oxygen species has several roles in cell signaling, cell communication, and homeostasis from bacteria to mammals. The ROS balance is maintained by the antioxidative property of cells. Disturbance or imbalance of this state leads to the oxidative stress of the cells. Oxidative stress has significant harmful effects on the cells due to the chemical reactions of ROS molecules with lipids and proteins. The high level or percentage of reactive oxygen molecules more than the threshold limit is toxic for the organisms and can have severe health consequences. Reactive oxygen and nitrogen species are toxic for bacteria causing illnesses in fish. Reactive oxygen species produced by neutrophils and macrophages could kill bacteria and, thus, constitute a primary element of non-specific defense in fish (Alexander et al. 2010)
Treatment
After 15 days of exposing the fishes to the extract in the tank water, the survival percentage of fishes in the second and third group after challenging them with E. tarda was recorded. Relative percentage survival (RPS) was found to be maximum (90%) in group 2 treated with the extract and injected with the bacteria. The RPS in group 3 which was not treated with the extract and injected with the bacteria was found to be low (20%). In group 3, clinical symptoms of loss of pigmentation, exophthalmia, and hemorrhage of fins were observed. In group 1, no mortality was observed. The results are given in Fig. 1
Identification of bioactive compounds using gas chromatography-mass spectrometry (GC-MS)
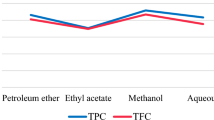
The compounds present in the petroleum ether extract and ethanolic extract of T. indica were analyzed to identify the chemical compounds present in them and also their respective peak areas. The bioactive molecules were found to be responsible for various properties like antibacterial, anti-tumor, anticancer, etc. which are attributed to them according to Dr. Duke’s database (Table 1). In the present study, the compounds 2-propanethioamide,3-(acetyloxy)-n,n-dimethyl-, (e), butanoic acid, ethyl ester, L-Isoasparagine, and N-hydroxyacetamide were identified from the petroleum ether extract, and (S)-3,4-dimethylpentanol, 2-pyrrolidine carboxamide,5-oxo,-(s), E-3-pentadecen-2-ol, and E-2-tetradecen-1-ol were identified from the ethanolic extract of T. indica seeds. Butanoic acid inhibits the production of uric acid. Uric acid is the end product of purine metabolism in humans and is an alternative physiological substrate for myeloperoxidase. Oxidation of uric acid by this enzyme generates uric acid free radical and urate hydroperoxide, a strong oxidant and potentially bactericide agent (Larissa Carvalho et al. 2018).
Biochemical test
The essential nutritional supplements for all organisms are carbohydrates, glucose, glycerides, and fatty acid and lipids. In this study, we have carried out the estimation of amino acids, carbohydrates, catalase, free fatty acids, glucose, glutathione S-transferase (GST), lipid peroxidation (LPO), total protein, superoxide dismutase (SOD), total cholesterol, and triglycerides contents in the healthy (control), infected, and treated groups. The levels of glucose and triglycerides and carbohydrate were found to be comparatively higher in the treated group. The level of these contents was comparatively decreased in the infected fishes. Fish are susceptible to the attack of ROS and have developed antioxidant defenses established by research primarily dating to the 1980s. Specially adapted enzymes, such as SOD, CAT, and enzymes dependent on glutathione (glutathione peroxidase and glutathione reductase, GST, reduced glutathione, and lipid peroxidation (LPO)) have been detected in most fish species investigated to date (Rudneva 1997). Ozcan Oruc et al. (2004) indicated that fish developed tissue-specific adaptive responses to protect cells against oxidative stress, with the gills showed increased level of change in SOD activity. Mbokane et al. (2018) reported that there was an increase in AST, ALT, ALP, and LDH in Oreochromis mossambicus when treated with Moringa oleifera-based diet (3, 6, and 9%) after experimental infection with Aeromonas hydrophila. Thanigaivel et al. (2019) have shown that the antioxidant response of Oreochromis mossambicus to a bacterial infection with Aeromonas is improved in fish fed with microencapsulated extracts of Gracilaria foliifera and Sargassum longifolium. The results are given in Fig. 2(Abutbul et al. 2004) to Fig. 2(Esau M. and Ngonidzashe A.G. 2018).
Antioxidant activity of different organs of fish Oreochromis mossambicus. (1) Amino acids, (2) carbohydrate, (3) catalase, (4) free fatty acids, (5) glucose, (6) glutathione S-transferase (GST), (7) lipid peroxidase (LPO), (8) protein, (9) SOD, (10) total cholesterol, (11) triglycerides. * indicates P value <0.05, ** indicate P value <0.005, *** indicate P<0.0005, ns indicates no significance difference between the groups
Immunological parameters
The TLC count in the normal fish was determined and was found to be 19±1.2 × 104 cells/mm3. The total erythrocyte count in normal fish was also determined and was found to be 20±2.0 × 107 cells/mm3. However, in the infected fish, the total leucocyte count was found to be 12±2.4 × 102 cells/mm3. The total erythrocyte count was found to be 15±1.0 × 107 cells/mm3. In the treated fish, the count was found to be near normal total leucocyte count 17±2.6 × 104 cells/mm3, while total erythrocyte count was found to be 18±2.0 × 107 cells/mm3. P value was found to be <0.05. Prabakaran et al. (2007) reported that in O. mossambicus, there was significant reduction of lysozyme activity and ROS on 32, 36, and 40 days in the group exposed to 0.053 and 0.53% of tannery effluent. The results are given in Fig. 3.
Conclusion
Spectroscopy of the extracts revealed the presence of numerous bioactive compounds in them. These compounds are reported to have antimicrobial, anticancer, and anti-inflammatory properties and also many essential medicinal uses. In vitro antimicrobial activity by well diffusion method was also found. The extract shows the presence of many phytocomponents such as flavonoids, saponin, carbohydrate, quinones, coumarins, and alkaloids. These secondary metabolites have insecticidal properties and also protect the plant from disease-causing organisms. Since the compounds in the extract have antimicrobial activity, they could be used as a prophylactic to prevent diseases in fishes, thus preventing the development of antibiotic resistance. We suggest that the active ingredients in T. indica extracts exhibiting antimicrobial properties in vitro should be isolated and subjected to further in vivo clinical trials and screening.
References
Abutbul S, Golan-Goldhirsh A, Barazani O, Zilberg D (2004) Use of Rosmarinus officinalis as a treatment against Streptococcus iniae in tilapia (Oreochromis sp.). Aquaculture 238:97–105
Alexander CP, Kirubakaran CJW, Michael RD (2010) Water soluble fraction of Tinospora cordifolia leaves enhanced the non-specific immune mechanisms and disease resistance in Oreochromis mossambicus. Fish Shellfish Immunol 29:765–772
Amend DF (1981) Potency testing of fish vaccines. Fish Biologics: Serodiagnostics and Vaccines 49:447–454
Aotsuka S, Okawa M, Ikebe K, Yokohari R (1979) Measurement of anti-double-stranded DNA antibodies in major immunoglobulin classes. J Immunol Methods 28(1-2):149–162
Ayalew A, Fufa A (2018) Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish. Vet Med Int 1–10
Beauchamp, Fridovich (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Berges Manual of Systemic Bacteriology (1984) The archaea and the deeply branching and phototrophic bacteria. Williams & Wilkins
Chandrasekhar Reddy B, Noor A, Sarada NC, Vijayalakshmi MA (2011) Antioxidant properties of Cordyline terminalis (L.) Kunth and Myristica fragrans Houtt encapsulated separately into casein beads. Curr Sci 101(3):416–420
Ewing WH, McWhorter AC, Escobar MR, Lubin AH (1965) Edwardsiella, a new genus of Enterobacteriaceae based on a new species, E tarda. Int J Syst Evol Microbiol 15(1):33–38
H.W. D, H.M. T Jr, R. M (1980) Experimental pathogenicity of Vibrio parahaemolyticus for the schistosome bearing snail Biomphalaria glabrata. Can J Microbial 26:503–506
Hedge JE, Hofreiter BT (1951) Carbohydrate chemistry. In: Whistler RL, Be Miller JN (eds) Academic Press, New York
Hoshina T (1962) On a new bacterium, paracolobactrumanguillimortiferum n. sp. Bull Jpn Soc Sci Fish 28:162–164
Janda, Abbott (1993) Unusual food borne pathogens Listeria monocytogenes, Aeromonas, Plesiomonas and Edwardsiella species. Clin Lab Med 19:553–582
Janda JM, Abbot SL, Krosle-Bystrom S, Cheung WK, Powers C, Kokka RP, Tamura K (1991) Pathogenic properties of Edwardsiella tarda species. J Clin Microbiol 29:1997–2001
Jaruratanasirikul, Kalnauwakul (1991) Edwardsiella tarda : a causative infection in human infections. Southeast Asian J Trop Med Public Health 22:30–40
Kubilay AS, Ulukoy AG, Ekici S, Dile O (2008) Immunization of rainbow trout (Oncorhynchus mykiss) against lactococcus garvieae using vaccine mixtures. Isr J Aquacult-Bamid 60(4):268–273
Larissa Carvalho AC, Lopes JPPB, Kaihami GH, Silva RP, Bruni-Cardoso A, Baldini RL, Meotti FC (2018) Uric acid disrupts hypochlorous acid production and the bactericidal activity of HL-60 cells. Redox Biol 16:179–188
Logambal SM, Venkatalakshmi S, Dinakaran Michael R (2000) Immunostimulatory effect of leaf extract of Ocimum sanctum Linn. In Oreochromis mossambicus (Peters). Hydrobiologia 430:113–120
Lowell BF, Ralph TD (1973) Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin Chem 19(3):338–340. https://doi.org/10.1093/clinchem/19.3.338
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mbokane EM, Moyo NAG (2018) Alterations of haemato-biochemical parameters pre and post-challenge with Aeromonas hydrophila and survival of Oreochromis mossambicus fed Moringa oleifera-based diets. Fish Shellfish Immunol 83:213–222
Meyer FP, Bullock GL (1973) Edwardsiella tarda, a new pathogen of channel catfish (Ictalurus punctatus). Appl Microbiol 25:155–156
Mohanty BR, Sahoo PK (2007) Edwardsiellosis in fish: a brief review. J Biosci 32:1331–1344
Montoro P, Tuberoso CI, Piacente S, Perrone A, De Feo V, Cabras P, Pizza C (2006) Stability and antioxidant activity of polyphenols in extracts of Myrtuscommunis L. berries used for the preparation of myrtle liqueur. J Pharm Biomed Anal 41(5):1614–1619
Nougayrede PH, Vuillaume A, Vigneulle M, Faivre B, Luengo S, Delprat J (1994) First isolation of Edwardsiella tarda from diseased turbot (Scophthalmus maximus) reared in a sea farm in the Bay of Biscay. Bull Eur Assoc Fish Pathol 14:128–129
Okawa H, Ohishi N, Ygi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Biochem 351–358
Ozcan Oruc E, Sevgiler Y, Uner N (2004) Tissue-specific oxidative stress responses in fish exposed to 2,4-D and azinphosmethyl. Comp Biochem Physiol 137:43–51
Pachanawan A, Phumkhachorn P, Rattanachaikunsopon P (2008) Potential of Psidiumguajava supplemented fish diets in controlling Aeromonas hydrophila infection in tilapia (Oreochromis niloticus). J Biosci Bioeng 106:419–424
Perez EE, Lewis EE (2006) Use of entomopathogenic nematodes and thyme oil to suppress plant-parasitic nematodes on English boxwood. Plant Dis 90(4):471–475
Plumb JA (1993) Edwardsiella septicaemia. In: Inglis V, Roberts RJ, Bromage NR, (eds) Bacterial diseases of fish. Blackwell Scientific, Oxford pp 61–79
Prabakaran M, Binuramesh C, Dieter Steinhagen R, Dinakaran Michael R (2007) Immune response in the tilapia Oreochromis mossambicus on exposure to tannery effluent. Ecotoxicol Environ Saf 68:372–378
Rattanachaikunsopon P, Phumkhachorn P (2007) Bacteriostatic effect of flavonoids isolated from leaves of Psidium guajava on fish pathogens. Fitoterapia 78:434–436
Rattanachaikunsopon P, Phumkhachorn P (2009) Prophylactic effect of Andrographis paniculata extracts against Streptococcus agalactiae infecting Nile tilapia (Oreochromis niloticus). J Biosci Bioeng 107:579–582
Rudneva II (1997) Blood antioxidant system of Black Sea elasmobranch and teleost. Comp Biochem Physiol 118C:255–260
Secombes CJ (1990) Isolation of salmonid macrophages and analysis for their activity. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB (Eds) Techniques in Fish Immunology. SOS Publications, New Jersey, pp 137–152
Singh MB, Bhalla PL, Malik CP (1980) Activity of some hydrolytic enzymes in autolysis of the embryo suspensor in Tropaeolum majus L. Ann Bot 45(5):523–527
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394
Šípová H, Ševců M, Kuchař V, Jawid N, Ahmad Mikulecký P, Šebo P, Malý J, Homola P (2011) Sensitive detection of interferon-gamma with engineered proteins and surface plasmon resonance biosensor. Procedia Eng 25:940–943
Thanigaivel S, Vijayakumar S, Mukherjee A, Chandrasekaran N, Thomas J (2014) Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture 433:467–475
Thanigaivel S, Vijayakumar S, Gopinath S, Mukherjee A, Chandrasekaran N, Thomas J (2015) In vivo and in vitro antimicrobial activity of Azadirachta indica (Lin) against Citrobacter freundii isolated from naturally infected Tilapia (Oreochromis mossambicus). Aquaculture 437:252–255
Thanigaivel S, Chandrasekaran N, Mukherjee A, Thomas J (2019) Protective efficacy of microencapsulated seaweed extracts for preventing Aeromonas infections in Oreochromis mossambicus. Comp Biochem Physiol C Toxicol Pharmacol 218:36–45
Thomas J, Jerobin J, Jeba S, Seelan T, Thanigaivel S, Vijaykumar S, Mukherjee A, Chandrasekharan N (2013a) Studies on the pathogenicity of Aeromonas salmonicida in catfish Clarias batrachus and control measures by neem nanoemulsion. Aquaculture 396-399:71–75
Thomas J, Madan N, Nambi KSN, Abdul Majeed S, Nazeer Basha A, Sahul Hameed AS (2013b) Studies on ulcerative disease caused by Aeromonas caviae-like bacterium in Indian catfish, Clarias batrachus (Linn). Aquaculture:376–379
Thomas J, Thanigaivel S, Vijayakumar S, Acharya K, Shinge D, Seelan TSJ, Mukherjee A, Chandrasekaran N (2014) Pathogenicity of Pseudomonas aeruginosa in Oreochromis mossambicus and treatment using lime oil nanoemulsion. Colloids Surf B: Biointerfaces 116:372–377
Wang H, James A. J (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27(5-6):612–616
William HH, Michael JP, William HJ (1974) Glutathione S-Transferases. J Biol Chem 249(22):7130–7139
Xu T, Zhang XH (2014) Edwardsiella tarda: an intriguing problem in aquaculture. Aquaculture 431:129–135
Yasunaga N, Ogawa S, Hatai K (1982) Characteristics of the fish pathogen Edwardsiella isolated from several species of cultured marine fishes. Bull Nagasaki Perfect Inst Fish 8:57–65
Acknowledgements
The authors thank the management of VIT, Vellore, for providing facilities to carry out the research work.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seth, M., Chandrasekaran, N., Mukherjee, A. et al. Pathogenicity of Edwardsiella tarda in Oreochromis mossambicus and treatment by Tamarindus indica seed extract. Aquacult Int 29, 1829–1841 (2021). https://doi.org/10.1007/s10499-021-00719-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00719-0