Abstract
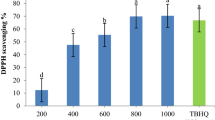
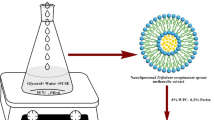
In the present study, the antioxidant activity of the extracts of Fumaria parviflora L. root and leaves obtained by ultrasound-assisted, microwave-assisted, supercritical fluid, and subcritical water extraction was assayed through DPPH radical scavenging method, ferric reduction assay, β-carotene: linoleic acid bleaching assay, and rancimat. The higher phenolic (50.47 mg GA/g DM) and flavonoid content (13.14 mg QE/g DM) was observed in ultrasonic leaves extract. The concentration-dependent antioxidant activity was observed for all extracts in all evaluation methods. The ultrasonic extract of F. parviflora L. leaves was incorporated into nanocapsules which were prepared with maltodextrin (MD), and gum Arabic (GA) at different ratio (1:0, 0:1, 1:1). The antioxidant activity of nano encapsulated extract was evaluated in sunflower oil (SFO) during 50 days of storage. Howbeit, the MD-GA nanocapsule was smaller than other nanocapsules, they had higher antioxidant properties in SFO. At the end of storage time, the lowest peroxide value (9.28 meq O2 kg−1 oil) and thiobarbituric acid value (4.34 mg malondialdehyde eq kg−1 oil) were related to oil containing MD-GA nanocapsules in comparison with control sample which exhibited the highest peroxide value (94.03 meq O2 kg−1 oil) and thiobarbituric acid value (0.32 mg malondialdehyde eq kg−1 oil) values. Also, the tert-Butylhydroquinone (TBHQ) had higher antioxidant capacity at lower concentration in all antioxidant tests, the SFO samples containing nanoencapsulated extract were more resistant to oxidation than control and TBHQ samples. The results of this study suggest that encapsulated F. parviflora L. leaves extract can be used as natural antioxidant to preserve sunflower oil.



Similar content being viewed by others
References
W. Rizvi, M. Fayazuddin, O. Singh, S.N. Syed, S. Moin, K. Akhtar, A. Kumar, Anti-inflammatory effect of Fumaria parviflora leaves based on TNF-α, IL-1, IL-6 and antioxidant potential. Avicenna. J. Phytomed. 7, 37–45 (2017)
M. Shokoohi, H. Shoorei, M. Soltani, S.H. Abtahi-Eivari, R. Salimnejad, M. Moghimian, Protective effects of the hydroalcoholic extract of Fumaria parviflora on testicular injury induced by torsion/detorsion in adult rats. Andrologia 50, e13047 (2018). https://doi.org/10.1111/and.13047
S. Kumar, A. Kamboj, A.K. Sharma, Antioxidant evaluation of ethanolic extract of Fumaria parviflora Lam. obtained from root, stem, leaf and fruit and measurement of their total phenols and flavonoids. Pharm. Innov. J. 7, 577–579 (2018)
R.E. Kenari, Z.R. Amiri, A. Motamedzadegan, J.M. Milani, J. Farmani, R. Farahmandfar, Optimization of Iranian golpar (Heracleum persicum) extract encapsulation using sage (Salvia macrosiphon) seed gum: chitosan as a wall materials and its effect on the shelf life of soybean oil during storage. J. Food. Meas. Charact. 14, 2828–2839 (2020). https://doi.org/10.1007/s11694-020-00528-8
C.S. Dzah, Y. Duan, H. Zhang, D.A. Authur, H. Ma, Ultrasound-, subcritical water-and ultrasound assisted subcritical water-derived Tartary buckwheat polyphenols show superior antioxidant activity and cytotoxicity in human liver carcinoma cells. Food. Res. Int. 137, 109598 (2020). https://doi.org/10.1016/j.foodres.2020.109598
S.S. Tometri, M. Ahmady, P. Ariaii, M.S. Soltani, Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. J. Food. Meas. Charact. 14, 3333–3344 (2020). https://doi.org/10.1007/s11694-020-00578-y
S. Belgheisi, A. Motamedzadegan, J.M. Milani, L. Rashidi, A. Rafe, Impact of ultrasound processing parameters on physical characteristics of lycopene emulsion. J. Food. Sci. Technol. 58, 484–493 (2020). https://doi.org/10.1007/s13197-020-04557-5
Y. Hu, Y. Li, W. Zhang, G. Kou, Z. Zhou, Physical stability and antioxidant activity of citrus flavonoids in arabic gum-stabilized microcapsules: modulation of whey protein concentrate. Food. Hydrocol. 77, 588–597 (2018). https://doi.org/10.1016/j.foodhyd.2017.10.037
B. Akdeniz, G. Sumnu, S. Sahin, The effects of maltodextrin and gum arabic on encapsulation of onion skin phenolic compounds. Chem. Eng. Trans. 57, 1891–1896 (2017). https://doi.org/10.3303/CET1757316
G.N. Mezza, A.V. Borgarello, N.R. Grosso, H. Fernandez, M.C. Pramparo, M.F. Gayol, Antioxidant activity of rosemary essential oil fractions obtained by molecular distillation and their effect on oxidative stability of sunflower oil. Food. Chem. 242, 9–15 (2018). https://doi.org/10.1016/j.foodchem.2017.09.042
M. Roostaee, M. Barzegar, M.A. Sahari, Z. Rafiee, The enhancement of pistachio green hull extract functionality via nanoliposomal formulation: studying in soybean oil. J. Food. Sci. Technol. 54, 3620–3629 (2017). https://doi.org/10.1007/s13197-017-2822-2
A. Salami, N. Asefi, R.E. Kenari, M. Gharekhani, Addition of pumpkin peel extract obtained by supercritical fluid and subcritical water as an effective strategy to retard canola oil oxidation. J. Food. Meas. Charact. 14, 2433–2442 (2020). https://doi.org/10.1007/s11694-020-00491-4
D. Chatterjee, P. Bhattacharjee, Comparative evaluation of the antioxidant efficacy of encapsulated and un-encapsulated eugenol-rich clove extracts in soybean oil: shelf-life and frying stability of soybean oil. J. Food. Eng. 117, 545–550 (2013). https://doi.org/10.1016/j.jfoodeng.2012.11.016
R. Esmaeilzadeh Kenari, F. Mohsenzadeh, Z.R. Amiri, Antioxidant activity and total phenolic compounds of Dezful sesame cake extracts obtained by classical and ultrasound-assisted extraction methods. Food. Sci. Nutr. 2, 426–435 (2014). https://doi.org/10.1002/fsn3.118
I.E. Orhan, N. Ozturk, B. Sener, Antiprotozoal assessment and phenolic acid profiling of five Fumaria (fumitory) species. Asian. Pac. J. Trop. Med. 8, 283–286 (2015). https://doi.org/10.1016/S1995-7645(14)60331-X
F. Simoes Grilo, Y. Srisaard, S.C. Wang, Prediction of walnut deterioration using kernel oxidative stability. Foods 9, 1207 (2020). https://doi.org/10.3390/foods9091207
I.E. Orhan, B. Şener, S.G. Musharraf, Antioxidant and hepatoprotective activity appraisal of four selected Fumaria species and their total phenol and flavonoid quantities. Exptl. Toxicol. Pathol. 64, 205–209 (2012). https://doi.org/10.1016/j.etp.2010.08.007
I. Ivanov, R. Vrancheva, A. Marchev, N. Petkova, I. Aneva, P. Denev, V.G. Georgiev, A. Pavlov, Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int. J. Curr. Microbiol. App. Sci. 3, 296–306 (2014)
M.S. Pak-Dek, A. Osman, N.G. Sahib, N. Saari, M. Markom, A.A. Hamid, F. Anwar, Effects of extraction techniques on phenolic components and antioxidant activity of Mengkudu (Morinda citrifolia L.) leaf extracts. J. Med. Plant. Res. 5, 5050–5057 (2011). https://doi.org/10.5897/JMPR.9000521
M. Co, A. Fagerlund, L. Engman, K. Sunnerheim, P.J. Sjöberg, C.J.P. Turner, Extraction of antioxidants from spruce (Picea abies) bark using eco-friendly solvents. Phytochem. Anal. 23, 1–11 (2012). https://doi.org/10.1002/pca.1316
L.G.G. Rodrigues, S. Mazzutti, I. Siddique, M. da Silva, L. Vitali, S.R.S. Ferreira, Subcritical water extraction and microwave-assisted extraction applied for the recovery of bioactive components from Chaya (Cnidoscolus aconitifolius Mill.). J. Supercrit. Fluids. 165, 104976 (2020). https://doi.org/10.1016/j.supflu.2020.104976
K.H. Lee, J.-S. Lee, E.S. Kim, H.G. Lee, Preparation, characterization, and food application of rosemary extract-loaded antimicrobial nanoparticle dispersions. LWT 101, 138–144 (2019). https://doi.org/10.1016/j.lwt.2018.10.072
I.T. Karabegović, S.S. Stojičević, D.T. Veličković, Z.B. Todorović, N.Č Nikolić, M.L. Lazić, The effect of different extraction techniques on the composition and antioxidant activity of cherry laurel (Prunus laurocerasus) leaf and fruit extracts. Ind. Crops Prod. 54, 142–148 (2014). https://doi.org/10.1016/j.indcrop.2013.12.047
D.I. Díaz, C.I. Beristain, E. Azuara, G. Luna, M. Jimenez, Effect of wall material on the antioxidant activity and physicochemical properties of Rubus fruticosus juice microcapsules. J. Microencapsul. 32, 247–254 (2015). https://doi.org/10.3109/02652048.2015.1010458
L.F. Ballesteros, M.J. Ramirez, C.E. Orrego, J.A. Teixeira, S.I. Mussatto, Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food. Chem. 237, 623–631 (2017). https://doi.org/10.1016/j.foodchem.2017.05.142
M.J. Ramírez, G.I. Giraldo, C.E. Orrego, Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder. Technol. 277, 89–96 (2015). https://doi.org/10.1016/j.powtec.2015.02.060
S.A. Mahdavi, S.M. Jafari, E. Assadpoor, D. Dehnad, Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 85, 379–385 (2016). https://doi.org/10.1016/j.ijbiomac.2016.01.011
Y.R.R.S. Rezende, J.P. Nogueira, N. Narain, Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: chemical, morphological and chemometric characterization. Food. Chem. 254, 281–291 (2018). https://doi.org/10.1016/j.foodchem.2018.02.026
A. Kalušević, S. Lević, B. Čalija, M. Pantić, M. Belović, V. Pavlović, B. Bugarski, J. Milić, S. Žilić, V. Nedović, Microencapsulation of anthocyaninrich black soybean coat extract by spray drying using maltodextrin, gum Arabic and skimmed milk powder. J. Microencapsul. 34, 475–487 (2017). https://doi.org/10.1080/02652048.2017.1354939
E. Anwar, N. Farhana, Formulation and evaluation of phytosome-loaded maltodextrin-gum arabic microsphere system for delivery of camellia sinensis extract. J. Young. Pharm. 10, S56 (2018). https://doi.org/10.5530/jyp.2018.2s.11
J.F. Gomes, S. Rocha, M. do Carmo Pereira, I. Peres, S. Moreno, J. Toca-Herrera, M.A. Coelho, , Lipid/particle assemblies based on maltodextrin–gum arabic core as bio-carriers. Colloids. Surf. B 76, 449–455 (2010). https://doi.org/10.1016/j.colsurfb.2009.12.004
K.M. Khazaei, S. Jafari, M. Ghorbani, A.H. Kakhki, Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydr. Polym. 105, 57–62 (2014). https://doi.org/10.1016/j.carbpol.2014.01.042
U. Bazylińska, D. Wawrzyńczyk, J. Kulbacka, R. Frąckowiak, B. Cichy, A. Bednarkiewicz, M. Samoć, K. Wilk, Polymeric nanocapsules with upconverting nanocrystals cargo make ideal fluorescent bioprobes. Sci. Rep. 6, 1–14 (2016). https://doi.org/10.1038/srep29746
F. Royshanpour, J. Tavakoli, F. Beigmohammadi, S. Alaee, Improving antioxidant effect of phenolic extract of Mentha piperita using nanoencapsulation process. J. Food Meas. Charact. 15, 23–32 (2020). https://doi.org/10.1007/s11694-020-00606-x
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Razavi, R., Kenari, R.E. Antioxidant evaluation of Fumaria parviflora L. extract loaded nanocapsules obtained by green extraction methods in oxidative stability of sunflower oil. Food Measure 15, 2448–2457 (2021). https://doi.org/10.1007/s11694-021-00837-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00837-6