Abstract
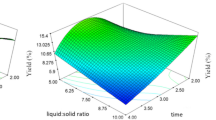
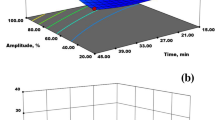
The microwave-assisted extractions (MAE) of Ocimum basilicum L. (basil) seed, Trigonella foenum-graecum (fenugreek) seed, and Plantago ovata Forsk (psyllium) seed husk hydrocolloids (BSH, FSH, and PSH, respectively) were performed at their maximum desirability function (for BSH) or optimum (for FSH and PSH) conditions and compared with conventional heating extraction (CHE). The extraction conditions had been defined based on two responses (Yield % and viscosity, mPa s) after relevant post-screening or optimization experiments based on previous experiments. Higher (17.89, 12.33, 32.60, 30.19, 71.73%) average extraction yields were obtained for BSH extracted by MAE (MAE-BSH), BSH extracted by CHE (CHE-BSH), FSH extracted by MAE (MAE-FSH), FSH extracted by CHE (CHE-FSH), and PSH extracted by MAE (MAE-PSH) in respective order compared with the estimated values (15.03, 9.96, 32, 22, and 65.4). Molecular weight (Mw) measurements were executed and the average Mw was determined to be 3.93 × 106, 2.89 × 106, 6.81 × 106 Da in the heavier fractions of BSH, FSH, and PSH. The FT-IR spectroscopy was performed and the predominant absorbance bands were defined. Dielectric properties of the 2% BSH, FSH, and PSH solutions were found to be comparable with distilled water. The steady flow analysis showed shear-thinning behavior for BSH, FSH, and PSH. The apparent viscosity (η, mPa s) of PSH was the highest followed by BSH and FSH. Dynamic flow measurements showed characteristics of a weak gel for BSH and PSH. Scanning electron microscopy showed morphological variations between the studied hydrocolloids. In addition, the study also indicated that microwave heating has lower destructive effects on the functional properties of the extract as compared to conventional thermal processing and could pave the way for future research. To conclude, MAE was found to be the efficient method for extraction of hydrocolloids with higher yield at a shorter extraction time.







Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Mathur, N.K.: General introduction to carbohydrates. In: Mathur, N.K. (Ed.) Industrial Galactomannan Polysaccharides, pp. 1–4. CRC Press, Boca Raton (2011)
Wielinga, W.C.: Galactomannans. In: Phillips, G.O.; Williams, P.A. (Eds.) Handbook of Hydrocolloids, pp. 228–251. Woodhead Publishing, Sawston (2009)
Mathur, N.K.: Galactomannan polysaccharides. In: Mathur, N.K. (Ed.) Industrial Galactomannan Polysaccharides, pp. 5–14. CRC Press, Boca Raton (2011)
Mirhosseini, H.; Amid, B.T.: A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res. Int. (2012). https://doi.org/10.1016/j.foodres.2011.11.017
Phillips, G.O.; Williams, P.A.: Introduction to food hydrocolloids. In: Phillips, G.O.; Williams, P.A. (Eds.) Handbook of Hydrocolloids, pp. 1–22. Woodhead Publishing, Sawston (2009)
Jackson, L.S.; Lee, K.: Microencapsulation and the food industry. Lebensm. Wiss. Technol. (1991). https://doi.org/10.1111/j.1745-4530.2008.00312.x
Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A.: In-vitro evaluation of hydrocolloid—based encapsulated fish oil. Food Hydrocoll. (2009). https://doi.org/10.1016/j.foodhyd.2008.10.009
Stijnman, A.C.; Bodnar, I.; Tromp, R.H.: Electrospinning of food-grade polysaccharides. Food Hydrocoll. (2011). https://doi.org/10.1016/j.foodhyd.2011.01.005
Mathur, N.K.: Interactions of galactomannans. In: Mathur, N.K. (Ed.) Industrial Galactomannan Polysaccharides, pp. 27–40. CRC Press, Boca Raton (2011)
Garti, N.; Madar, Z.; Aserin, A.; Sternheim, B.: Fenugreek galactomannans as food emulsifiers. LWT Food Sci. Technol. (1997). https://doi.org/10.1006/fstl.1996.0179
Youssef, M.; Wang, Q.; Cui, S.; Barbut, S.: Purification and partial physicochemical characteristics of protein free fenugreek gums. Food Hydrocoll. (2009). https://doi.org/10.1016/j.foodhyd.2009.03.017
Karazhiyan, H.; Razavi, S.M.; Phillips, G.O.: Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. (2011). https://doi.org/10.1016/j.foodhyd.2010.08.022
Koocheki, A.; Mortazavi, S.A.; Shahidi, F.; Razavi, S.; Kadkhodaee, R.; Milani, J.M.: Optimization of mucilage extraction from Qodume shirazi seed (Alyssum homolocarpum) using response surface methodology. J. Food Process. Eng. 33, 861 (2010). https://doi.org/10.1111/j.1745-4530.2008.00312.x
Keisandokht, S.; Haddad, N.; Gariepy, Y.; Orsat, V.: Screening the microwave-assisted extraction of hydrocolloids from Ocimum basilicum L. seeds as a novel extraction technique compared with conventional heating–stirring extraction. Food Hydrocoll. (2018). https://doi.org/10.1016/j.foodhyd.2017.07.016
Komarov, V.V.: Handbook of Dielectric and Thermal Properties of Materials at Microwave Frequencies. Artech House, Boston (2012)
Naji-Tabasi, S.; Razavi, S.M.A.; Mohebbi, M.; Malaekeh-Nikouei, B.: New studies on basil (Ocimum bacilicum L.) seed gum: part I-fractionation, physicochemical and surface activity characterization. Food Hydrocoll. (2016). https://doi.org/10.1016/j.foodhyd.2015.07.011
Brummer, Y.; Cui, W.; Wang, Q.: Extraction, purification and physicochemical characterization of fenugreek gum. Food Hydrocoll. (2003). https://doi.org/10.1016/S0268-005X(02)00054-1
Guo, Q.; Cui, S.W.; Wang, Q.; Young, J.C.: Fractionation and physicochemical characterization of psyllium gum. Carbohydr. Polym. (2008). https://doi.org/10.1016/j.carbpol.2007.11.001
Al-Assaf, S.; Phillips, G.O.; Williams, P.A.; Takigami, S.; Dettmar, P.; Havler, M.: Molecular weight, tertiary structure, water binding and colon behaviour of ispaghula husk fibre. Proc. Nutr. Soc. (2003). https://doi.org/10.1079/pns2002216
Yin, J.; Lin, H.; Li, J.; Wang, Y.; Cui, S.W.; Nie, S.; Xie, M.: Structural characterization of a highly branched polysaccharide from the seeds of Plantago asiatica L. Carbohydr. Polym. (2012). https://doi.org/10.1016/j.carbpol.2011.11.009
Robert, P.; Marquis, M.; Barron, C.; Guillon, F.; Saulnier, L.: FT-IR investigation of cell wall polysaccharides from cereal grains. Arabinoxylan infrared assignment. J. Agric. Food Chem. (2005). https://doi.org/10.1021/jf051145y
Kuptsov, A.H.; Zhizhin, G.N.: Handbook of Fourier Transform Raman and Infrared Spectra of Polymers. Elsevier, Amsterdam (1998)
Kurt, A.; Kahyaoglu, T.: Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. (2014). https://doi.org/10.1016/j.carbpol.2014.01.003
Zhang, H.; Yoshimura, M.; Nishinari, K.; Williams, M.; Foster, T.; Norton, I.: Gelation behaviour of konjac glucomannan with different molecular weights. Biopolymers (2001). https://doi.org/10.1002/1097-0282
Chua, M.; Chan, K.; Hocking, T.J.; Williams, P.A.; Perry, C.J.; Baldwin, T.C.: Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr. Polym. (2012). https://doi.org/10.1016/j.carbpol.2011.10.053
Cerqueira, M.; Bourbon, A.; Pinheiro, A.; Martins, J.; Souza, B.; Teixeira, J.; Vicente, A.: Galactomannans use in the development of edible films/coatings for food applications. Trends Food Sci. Technol. (2011). https://doi.org/10.1016/j.tifs.2011.07.002
Ahmed, J.; Ramaswamy, H.; Raghavan, G.: Dielectric properties of soybean protein isolate dispersions as a function of concentration, temperature and pH. LWT Food Sci. Technol. (2008). https://doi.org/10.1016/j.lwt.2007.01.017
Cristina, V.; Paolo, C.; Giancarlo, C.: Microwave-assisted extraction for bioactive compounds: an introduction to dielectric heating. In: Chemat, F.; Cravotto, G. (Eds.) Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice, pp. 1–14. Springer, Berlin (2013)
Dickinson, E.: Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. (2003). https://doi.org/10.1016/S0268-005X(01)00120-5
Farahnaky, A.; Askari, H.; Majzoobi, M.; Mesbahi, G.: The impact of concentration, temperature and pH on dynamic rheology of psyllium gels. J. Food Eng. (2010). https://doi.org/10.1016/j.jfoodeng.2010.04.012
Naji-Tabasi, S.; Razavi, S.M.A.: New studies on basil (Ocimum bacilicum L.) seed gum: part III—steady and dynamic shear rheology. Food Hydrocoll. (2017). https://doi.org/10.1016/j.foodhyd.2015.12.020
Rafe, A.; Razavi, S.: Dynamic viscoelastic study on the gelation of basil seed gum. Int. J. Food Sci. Technol. (2012). https://doi.org/10.1111/j.1365-2621.2012.03221.x
Urlacher, B.; Noble, O.: Xanthumgum. In: Imeson, A. (Ed.) Thickening and gelling agents for food, pp. 284–311. Springer, Berlin (1997)
Fox, J.E.: Seed gums. In: Imeson, A. (Ed.) Food stabilizers, thickeners, and gelling agents, pp. 153–170. Wiley-Blackwell, Hoboken (2009)
Zecher, D.; Gerrish, T.: Cellulose derivatives. In: Imeson, A. (Ed.) Thickening and Gelling Agents for Food, pp. 60–85. Springer, Berlin (1997)
Wielinga, W.C.: Seed gums. In: Phillips, G.O.; Williams, P.A. (Eds.) Handbook of Hydrocolloids, pp. 275–292. Woodhead Publishing, Sawston (2009)
Phillips, G.O.; Williams, P.A.: Gum arabic. In: Phillips, G.O.; Williams, P.A. (Eds.) Handbook of hydrocolloids, pp. 252–273. Woodhead Publishing, Sawston (2009)
Fadavi, G.; Mohammadifar, M.; Zargaran, A.; Azadnia, E.: The study of composition, molecular weight and rheological characteristics of Zedo gum exudates from Amygdalus scoparia. Iran. J. Nutr. Sci. Food Technol. 7, 35 (2013)
Golkar, A.; Nasirpour, A.; Keramat, J.; Desobry, S.: Emulsifying properties of Angum gum (Amygdalus scoparia Spach) conjugated to β-lactoglobulin through Maillard-type reaction. Int. J. Food Prop. (2015). https://doi.org/10.1080/10942912.2014.962040
Sanchez, C.; Renard, D.; Robert, P.; Schmitt, C.; Lefebvre, J.: Structure and rheological properties of acacia gum dispersions. Food Hydrocoll. (2002). https://doi.org/10.1016/S0268-005X(01)00096-0
Wareing, M.V.: Exudate gums. In: Imeson, A. (Ed.) Thickening and Gelling Agents for Food, pp. 86–118. Springer, Berlin (2009)
Jafari, S.M.; Beheshti, P.; Assadpoor, E.: Rheological behavior and stability of D-limonene emulsions made by a novel hydrocolloid (Angum gum) compared with Arabic gum. J. Food Eng. (2012). https://doi.org/10.1016/j.jfoodeng.2011.10.016
Bourbon, A.; Pinheiro, A.; Ribeiro, C.; Miranda, C.; Maia, J.; Teixeira, J.; Vicente, A.: Characterization of galactomannans extracted from seeds of Gleditsia triacanthos and Sophora japonica through shear and extensional rheology: comparison with guar gum and locust bean gum. Food Hydrocoll. (2010). https://doi.org/10.1016/j.foodhyd.2009.09.004
Guo, Q.; Cui, S.W.; Wang, Q.; Goff, H.D.; Smith, A.: Microstructure and rheological properties of psyllium polysaccaharide gel. Food Hydorcoll. (2009). https://doi.org/10.1016/j.foodhyd.2008.10.012
Rafe, A.; Razavi, S.M.; Farhoosh, R.: Rheology and microstructure of basil seed gum and β-lactoglobulin mixed gels. Food Hydrocoll. (2013). https://doi.org/10.1016/j.foodhyd.2012.05.016
Hesarinejad, M.A.; Koocheki, A.; Razavi, S.M.A.: Dynamic rheological properties of Lepidium perfoliatum seed gum: effect of concentration, temperature and heating/cooling rate. Food Hydrocoll. (2014). https://doi.org/10.1016/j.foodhyd.2013.07.017
Robinson, G.; Ross-Murphy, S.B.; Morris, E.R.: Viscosity-molecular weight relationships, intrinsic chain flexibility, and dynamic solution properties of guar galactomannan. Carbohydr. Res. (1992). https://doi.org/10.1016/S0008-6215(00)80772-7
Wei, Y.; Lin, Y.; Xie, R.; Xu, Y.; Yao, J.; Zhang, J.: The flow behavior, thixotropy and dynamical viscoelasticity of fenugreek gum. J Food Eng. (2015). https://doi.org/10.1016/j.jfoodeng.2015.05.015
Rahimi, S.; Abbasi, S.: Determination of some physicochemical properties and gelification of Persian gum. Sci. Technol. Nov. Foods 4, 13 (2014)
Smith, F.; Montgomery, R.: The Chemistry of Plant Gums and Mucilages. Reinhold Publishing Corporation, New York (1959)
Khazaei, N.; Esmaiili, M.; Djomeh, Z.E.; Ghasemlou, M.; Jouki, M.: Characterization of new biodegradable edible film made from basil seed (Ocimum basilicum L.) gum. Carbohydr. Polym. (2014). https://doi.org/10.1016/j.carbpol.2013.10.062
Herranz, B.; Borderias, A.J.; Solo-de-Zaldívar, B.; Solas, M.T.; Tovar, C.A.: Thermostability analyses of glucomannan gels. Concentration influence. Food Hydrocoll. (2012). https://doi.org/10.1016/j.foodhyd.2012.02.011
Pawar, H.A.; Lalitha, K.: Isolation, purification and characterization of galactomannans as an excipient from Senna tora seeds. Int. J. Biol. Macromol. (2014). https://doi.org/10.1016/j.ijbiomac.2014.01.026
Acknowledgements
Not applicable.
Funding
The financial support of the Natural Science and Engineering Research Council of Canada (NSERC) is acknowledged by the authors of this research paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Keisandokht, S., Orsat, V., Karboune, S. et al. Microwave-Assisted Extraction of Ocimum basilicum L. Seed, Trigonella foenum-graecum Seed, and Plantago ovata Forsk Seed Husk Hydrocolloids Compared with Conventional Heating Extraction at Optimum Extraction Conditions. Arab J Sci Eng 47, 5859–5874 (2022). https://doi.org/10.1007/s13369-021-05792-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05792-4