Abstract
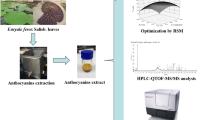
Betacyanin and betaxanthin are natural colouring compounds found in Amaranthus tricolour leaves. Microwave-assisted extraction was performed to extract betacyanin and betaxanthin from the unsalable leaves. A set of different process parameters like microwave power, temperature and time was applied in this study. The combination of 450-W microwave power, 90 °C temperature and 15 min of extraction time resulted in the highest amount of betacyanin (71.95 mg/g of dw) recovery. The highest amount of betaxanthin (42.30 mg/g of dw) was extracted at 200-W microwave power, 60 °C temperature and 15 min of extraction time. Green extraction was done by using water as the solvent based on the solubility of compounds being highest in it. The combined effect of time and temperature was also found to be significant for the extraction. The betacyanin and betaxanthin also exhibited good scavenging ability against superoxide and hydrogen peroxide. The recovery was maximum at higher temperatures with a shorter time of extraction. The SEM images showed that the plant matrix ruptured due to microwaves. The FTIR spectroscopy showed the existence of betacyanin and betaxanthin in the leaf extract. The microwave technique was also proved to be better than ultrasound extraction and Soxhlet extraction. The colour change in the powdered sample was observed after extraction which shows the efficient recovery of pigments. The A. tricolour leaves are the cheaper source of betacyanin and betaxanthin. These compounds not only possess colouring properties but also exhibit antioxidant and medicinal properties. Hence, betacyanin and betaxanthin extracted from these leaves can be used as additives and colourants in food products.











Similar content being viewed by others
References
Adeniyi SA, Ehiagbonare JE, Nwangwu SCO (2012) Nutritional evaluation of some staple leafy vegetables in Southern Nigeria. Int J Agric Food Sci 2:37–43
Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG (2018) Fruit and vegetable waste: bioactive compounds their extraction and possible utilization. Compr Rev Food Sci Food Saf 17:512–531. https://doi.org/10.1111/1541-4337.12330
Gogo EO, Opiyo AM, Ulrichs C, Huyskens-Keil S (2017) Nutritional and economic postharvest loss analysis of African indigenous leafy vegetables along the supply chain in Kenya Postharvest. Biol Technol 130:39–47. https://doi.org/10.1016/j.postharvbio.2017.04.007
Able AJ, Wong LS, Prasad A, O’Hare TJ (2003) The effects of 1-methylcyclopropene on the shelf life of minimally processed leafy asian vegetables. Postharvest Biol Technol 27:157–161. https://doi.org/10.1016/S0925-5214(02)00093-5
Ambuko J, Wanjiru F, Chemining’wa GN, Owino WO, Mwachoni E (2017) Preservation of postharvest quality of leafy Amaranth (Amaranthus spp.) vegetables using evaporative cooling. J Food Qual 2017:1–7. https://doi.org/10.1155/2017/5303156
Jacotet-Navarro M, Rombaut N, Deslis S, Fabiano-Tixier A, Pierre F, Bily A, Chemat F (2016) Towards a “dry” bio-refienry without solvent or added water using microwaves and ultrasound for total valorization of fruits and vegetables by-products Green Chem 18:3106–3115. https://doi.org/10.1039/C5GC02542G
Sarker U, Oba S (2018) Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem 252:72–83. https://doi.org/10.1016/j.foodchem.2018.01.097
Chun OK, Kim DO, Lee CY (2003) Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem 51:8067–8072. https://doi.org/10.1021/jf034740d
Kujala T, Loponen J, Pihlaja K (2001) Betalains and phenolics in red beetroot (Beta vulgaris) peel extracts: extraction and characterisation. Z Naturforsch C J Biosci 56:343–348. https://doi.org/10.1515/znc-2001-5-604
Butera D, Tesoriere L, Di Gaudio F, Bongiorno A, Allegra M, Pintaudi AM, Kohen R, Livrea MA (2002) Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: betanin and indicaxanthin. J Agric Food Chem 50:6895–6901. https://doi.org/10.1021/jf025696p
Harivaindaran KV, Rebecca OPS, Chandran S (2008) Study of optimal temperature, pH and stability of dragon fruit (Hylocereus polyrhizus) peel for use as potential natural colorant. Pak J Biol Sci 11:2259–2263. https://doi.org/10.3923/pjbs.2008.2259.2263
Azeredo HMC (2009) Betalains: properties, sources, applications, and stability - a review. Int J Food Sci Technol 44:2365–2376. https://doi.org/10.1111/j.1365-2621.2007.01668.x
Celli GB, Brooks MSL (2017) Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins — a current review. Food Res Int 100:501–509. https://doi.org/10.1016/j.foodres.2016.08.034
Zin MM, Marki E, Banvolgyi S (2020) Recovery pf phytochemicals via elctromagnetic irradiation (microwave-assisted-extraction): betalains and phenolic compounds in perspective. Foods 9:918. https://doi.org/10.3390/foods9070918
Kaufmann B, Christen P (2002) Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem Anal 13:105–113. https://doi.org/10.1002/pca.631
Dahmoune F, Nayak B, Moussi K, Remini H, Madani K (2015) Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem 166:585–595. https://doi.org/10.1016/j.foodchem.2014.06.066
Chen S, Zeng Z, Hu N, Bai B, Wang H, Suo Y (2018) Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem 242:1–8. https://doi.org/10.1016/j.foodchem.2017.08.105
Zin MM, Marki E, Banvolgyi S (2020) Conventional extraction of betalain compounds from beetroot peels with aqueous ethanol solvent. Acta Aliment 49:163–169. https://doi.org/10.1556/066.2020.49.2.5
Zin MM, Marki E, Banvolgyi S (2020) Evaluation of reverse osmosis membranes in concentration of beetroot peel extract. Period Polytech Chem Eng 64:340–348. https://doi.org/10.3311/PPch.15040
Alam MN, Bristi NJ, Rafiquzzaman M (2013) Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 21:143–152. https://doi.org/10.1016/j.jsps.2012.05.002
Fathordoobady F, Mirhosseini H, Selamat J, Manap MYA (2016) Effect of solvent type and ratio on betacyanins and antioxidant activity of extracts from Hylocereus polyrhizus flesh and peel by supercritical fluid extraction and solvent extraction. Food Chem 202:70–80. https://doi.org/10.1016/j.foodchem.2016.01.121
Reshmi SK, Aravinthan KM, Suganya devi P (2012) Antioxidant analysis of betacyanin extracted from Basella alba fruit. Int J PharmTech Res 4:900–913
Swamy GJ, Sangamithra A, Chandrasekar V (2014) Response surface modeling and process optimization of aqueous extraction of natural pigments from Beta vulgaris using Box-Behnken design of experiments. Dyes Pigments 111:64–74. https://doi.org/10.1016/j.dyepig.2014.05.028
Thirugnanasambandham K, Sivakumar V (2017) Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. J Saudi Soc Agric Sci 16:41–48. https://doi.org/10.1016/j.jssas.2015.02.001
Cardoso-Ugarte GA, Sosa-Morales ME, Ballard T, Liceaga A, San Martín-González MF (2014) Microwave-assisted extraction of betalains from red beet (Beta vulgaris). LWT Food Sci Technol 59:276–282. https://doi.org/10.1016/j.lwt.2014.05.025
Cai Y, Sun M, Wu H, Huang R, Corke H (1998) Characterization and quantification of betacyanin pigments from diverse Amaranthus species J Agric Food Chem 46. https://doi.org/10.1021/jf9709966
Syafinar R, Gomesh N, Irwanto M, Fareq M, Irwan YM (2015) FT-IR and UV-VIS spectroscopy photochemical analysis of dragon fruit. ARPN J Eng Appl Sci 10:6354–6358
Adedokun O, Sanusi YK, Awodugba AO (2018) Solvent dependent natural dye extraction and its sensitization effect for dye sensitized solar cells. Optik (Stuttg) 174:497–507. https://doi.org/10.1016/j.ijleo.2018.06.064
Melgar B, Dias MI, Barros L, Ferreira ICFR, Rodriguez-Lopez AD, Garcia-Castello EM (2019) Ultrasound and microwave assisted extraction of Opuntia fruit peels biocompounds: Optimization and comparison using RSM-CCD. Molecules 24:1–16. https://doi.org/10.3390/molecules24193618
Maran JP, Priya B (2015) Natural pigments extraction from Basella rubra L. fruits by ultrasound-assisted extraction combined with Box-Behnken response surface design. Sep Sci Technol 50:1532–1540. https://doi.org/10.1080/01496395.2014.980003
Ferreres F, Grosso C, Gil-Izquierdo A, Valentão P, Mota AT, Andrade PB (2017) Optimization of the recovery of high-value compounds from pitaya fruit by-products using microwave-assisted extraction. Food Chem 230:463–474. https://doi.org/10.1016/j.foodchem.2017.03.061
Singh N, Rajini PS (2004) Free radical scavenging activity of an aqueous extract of potato peel. Food Chem 85:611–616. https://doi.org/10.1016/j.foodchem.2003.07.003
Zhang J, Hou X, Ahmad H, Zhang H, Zhang L, Wang T (2014) Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem 145:57–65. https://doi.org/10.1016/j.foodchem.2013.08.025
Neelwarne B (2012) Red beet biotechnology: food and pharmaceutical applications Red Beet Biotechnol:1–435. https://doi.org/10.1007/978-1-4614-3458-0
Kaderides K, Papaoikonomou L, Serafim M, Goula AM (2019) Microwave-assisted extraction of phenolics from pomegranate peels: optimization, kinetics, and comparison with ultrasounds extraction. Chem Eng Process Process Intensif 137:1–11. https://doi.org/10.1016/j.cep.2019.01.006
Coy-Barrera E (2020) Analysis of betalains (betacyanins and betaxanthins) Recent Adv Nat Prod Anal 593–619. https://doi.org/10.1016/b978-0-12-816455-6.00017-2
Šeremet D, Durgo K, Jokić S, Hudek A, Cebin AV, Mandura A, Jurasović J, Komes D (2020) Valorization of banana and red beetroot peels: determination of basic macrocomponent composition, application of novel extraction methodology and assessment of biological activity in vitro. Sustain 12:4539. https://doi.org/10.3390/su12114539
Zhongdong L, Guohua W, Yunchang G, Kennedy JF (2006) Image study of pectin extraction from orange skin assisted by microwave. Carbohydr Polym 64:548–552. https://doi.org/10.1016/j.carbpol.2005.11.006
Author information
Authors and Affiliations
Contributions
The experiments and analysis were performed by Alok Sharma. The research was planned and designed by Bidyut Mazumdar. The research paper was written and drafted by Amit Keshav.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, A., Mazumdar, B. & Keshav, A. Valorization of unsalable Amaranthus tricolour leaves by microwave-assisted extraction of betacyanin and betaxanthin. Biomass Conv. Bioref. 13, 1251–1267 (2023). https://doi.org/10.1007/s13399-020-01267-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01267-y