Abstract
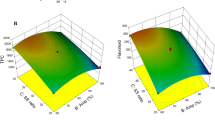
Citrus reticulata (kinnow) peel is a good source of carotenoids which can be extracted using ultrasound-assisted extraction (UAE). The present study was conducted to optimize the process parameters for the extraction of lutein using UAE method with methanolic solution of potassium hydroxide as the extractant to obtain its maximum yield. The obtained extract was used for the determination of lutein yield using high-pressure liquid chromatography (HPLC). The optimized values of process parameters in the current investigation were 6.16 mL/g solvent/solid ratio, 43.14 °C temperature, 32.88% amplitude and time duration of 33.71 min, which resulted in highest lutein yield of 29.70 μg/g. The results of this study showed that UAE system has the ability to replace the conventional techniques for extraction of lutein from citrus peels. Moreover, the extracted lutein from citrus peels can be further used as an antioxidant and colouring agent in various food applications.







Similar content being viewed by others
References
Devkota RP, Grewal SS, Dhatt AS (1982) Maturity determination in kinnow mandarin. Punjab Hort J 22:131–135
Rafiq S, Kaul R, Sofi SA, Bashir N, Nazir F, Ahmad Nayik G (2018) Citrus peel as a source of functional ingredient: a review. J Saudi Soc Agric Sci 17:351–358
Saini A, Panesar PS, Bera MB (2019) Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour Bioprocess 6:1–12. https://doi.org/10.1186/s40643-019-0261-9
Chhikara N, Kour R, Jaglan S, Gupta P, Gat Y, Panghal A (2018) Citrus medica: nutritional, phytochemical composition and health benefits—a review. Food Funct 9:1978–1992
Xu CJ, Fraser PD, Wang WJ, Bramley PM (2006) Differences in the carotenoid content of ordinary citrus and lycopene-accumulating mutants. J Agric Food Chem 54:5474–5481
Yen WJ, Chen BH (1995) Isolation of xanthophylls from Taiwanese orange peels and their effects on the oxidation stability of soybean oil. Food Chem 53:417–425
Fernandez-Sevilla JM, Acien Fernandez FG, Molina Grima E (2010) Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 86:27–40
Lin JH, Lee DJ, Chang JS (2015) Lutein production from biomass: marigold flowers versus microalgae. Bioresour Technol 184:421–428
Del Campo JA, Garcia-Gonzalez M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
Wilkins MR, Suryawati L, Maness NO, Chrz D (2007) Ethanol production by Saccharomyces cerevisiae and Kluyveromyces marxianus in the presence of orange-peel oil. World J Microbiol Biotechnol 23:1161–1168
FAO (2014) Definitional framework of food losses and waste. Rome
Panwar D, Panesar PS, Chopra HK (2019) Recent trends on the valorization strategies for the management of citrus by-products. Food Rev Int. https://doi.org/10.1080/87559129.2019.1695834
Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG (2018) Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf 17:512–531
Altemimi A, Lightfoot DA, Kinsel M, Watson DG (2015) Employing response surface methodology for the optimization of ultrasound assisted extraction of lutein and beta-carotene from spinach. Molecules 20:6611–6625
Fu XQ, Zhang G, Deng L, Dang YY (2018) Simultaneous extraction and enrichment of polyphenol and lutein from marigold (Tagetes erecta L.) flower by enzyme-assisted ethanol/ammonium sulfate system. Food Funct 10:66–276
Gayathri S, Rajasree Radhika SR, Suman TY, Aranganathan L (2018) Ultrasound-assisted microextraction of β, ε-carotene-3, 3′-diol (lutein) from marine microalgae Chlorella Salina: effect of different extraction parameters. Biomass Convers Bior 8:791–797
Cobb BF, Kallenbach J, Hall CA, Pryor SW (2018) Optimizing the supercritical fluid extraction of lutein from corn gluten meal. Food Bioprocess Technol 11:757–764
Drosou C, Kyriakopoulou K, Bimpilas A, Tsimogiannis D, Krokida M (2015) A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind Crop Prod 75:141–149
Farahmandfar R, Esmaeilzadeh Kenari R, Asnaashari M, Shahrampour D, Bakhshandeh T (2019) Bioactive compounds, antioxidant and antimicrobial activities of Arum maculatum leaves extracts as affected by various solvents and extraction methods. Food Sci Nutr 7:465–475
Chemat F, Tomao V, Virot M (2008) Ultrasound-assisted extraction in food analysis. In: Ötleş S (ed) Handbook of food analysis instruments. CRC Press, Boca Raton, pp 85–103
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem 34:540–560
Garrido T, Gizdavic-Nikolaidis M, Leceta I, Urdanpilleta M, Guerrero P, De la Caba K, Kilmartin PA (2019) Optimizing the extraction process of natural antioxidants from chardonnay grape marc using microwave-assisted extraction. Waste Manag 88:110–117
Wang B, Hui Y, Liu L, Zhao A, Chiou YS, Zhang F, Pan MH (2019) Optimized extraction of phenolics from jujube peel and their anti-inflammatory effects in LPS-stimulated murine macrophages. J Agric Food Chem 67:1666–1673
Derrien M, Badr A, Gosselin A, Desjardins Y, Angers P (2019) Optimization of a sustainable purification protocol for lutein and chlorophyll from spinach by-products by a saponification procedure using box Behnken design and desirability function. Food Bioprod Process 116:54–62
Talib R, Syuhadah N, Halmi MIE, Abd Ghani SS, Zaidan UH, Shukor MYA (2019) Artificial neural networks (ANNs) and response surface methodology (RSM) approach for modelling the optimization of chromium (VI) reduction by ewly isolated Acinetobacter radioresistens strain NS-MIE from agricultural soil. Biomed Res Int. https://doi.org/10.1155/2019/5785387
Khan MK, Abert-Vian M, Fabiano-Tixier AS, Dangles O, Chemat F (2010) Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem 119:851–858
Safdar MN, Kausar T, Jabbar S, Mumtaz A, Ahad K, Saddozai AA (2017) Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J Food Drug Anal 25:488–500
Agócs A, Nagy V, Szabó Z, Márk L, Ohmacht R, Deli J (2007) Comparative study on the carotenoid composition of the peel and the pulp of different citrus species. Innov Food Sci Emerg Technol 8:390–394
Craft NE (2001) Chromatographic techniques for carotenoid separation. In: Giusti MM, Wrolstad RE (eds) Current protocols in food analytical chemistry. Wiley, Hoboken, pp F2.3.1–F2.3.15
Digesù AM, Platani C, Cattivelli L, Mangini G, Blanco A (2009) Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. J Cereal Sci 50:210–218
Varzakas T, Kiokias S (2016) HPLC analysis and determination of carotenoid pigments in commercially available plant extracts. Curr Res Nutr Food Sci 4:1–14
Xia EQ, Ai XX, Zang SY, Guan TT, Xu XR, Li HB (2011) Ultrasound-assisted extraction of phillyrin from Forsythia suspense. Ultrason Sonochem 18:549–552
Deng FG, Xu DP, Li S, Li HB (2015) Optimization of ultrasound-assisted extraction of natural antioxidants from sugar apple (Annona squamosa L.) peel using response surface methodology. Molecules 20:20448–20459
Santos HM, Lodeiro C, Capelo-Martínez JL (2009) The power of ultrasound. In: Capelo-Martínez JL (ed) Ultrasound in chemistry: analytical applications. Wiley-VCH Verlag, Weinheim, pp 1–16
Ranjan A, Patil C, Moholkar VS (2010) Mechanistic assessment of microalgal lipid extraction. Ind Eng Chem Res 49:2979–2985
Singh D, Barrow CJ, Mathur AS, Tuli DK, Puri M (2015) Optimization of zeaxanthin and β-carotene extraction from Chlorella saccharophila isolated from New Zealand marine waters. Biocatal Agric Biotechnol 4:166–173
Al-dhabi NA, Ponmurugan K, Maran P (2017) Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason Sonochem 34:206–213
Carrera C, Ruiz-rodríguez A, Palma M, Barroso CG (2012) Ultrasound assisted extraction of phenolic compounds from grapes. Anal Chim Acta 732:100–104
Tiwari H, Singh P, Mishra P, Srivastava P (2010) Evaluation of various techniques for extraction of natural colorants from pomegranate rind-ultrasound and enzyme assisted extraction. Indian J Fibre Text Res 35:272–276
Ghitescu RE, Volf I, Carausu C, Bühlmann AM, Gilca IA, Popa VI (2015) Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason Sonochem 22:535–541
Calvo MM (2005) Lutein: a valuable ingredient of fruit and vegetables. Crit Rev Food Sci Nutr 45:671–696
Ghafoor K, Choi YH, Jeon JY, Jo IH (2009) Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J Agric Food Chem 57:4988–4994
Tomšik A, Pavlić B, Vladić J, Ramić M, Brindza J, Vidović S (2016) Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason Sonochem 29:502–511
M’hiri N, Ioannou I, Boudhrioua NM, Ghoul M (2015) Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod Process 6:161–170
Wang YC, Chuang YC, Hsu HW (2008) The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem 106:277–284
Mehmood A, Ishaq M, Zhao L, Yaqoob S, Safdar B, Nadeem M, Munir M, Wang C (2019) Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower (Clitoria ternatea L.). Ultrason Sonochem 51:12–19
Saini A, Panesar PS, Bera MB (2019) Comparative study on the extraction and quantification of polyphenols from citrus peels using maceration and ultrasonic technique. Curr Res Nutr Food Sci. https://doi.org/10.12944/CRNFSJ.7.3.08
Song J, Yang Q, Huang W, Xiao Y, Li D, Liu C (2018) Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound-assisted extraction. Food Bioprod Process 107:104–112
Luengo E, Condón-Abanto S, Condón S, Álvarez I, Raso J (2014) Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep Purif Technol 136:130–136
Funding
This work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saini, A., Panesar, P.S. & Bera, M.B. Valuation of Citrus reticulata (kinnow) peel for the extraction of lutein using ultrasonication technique. Biomass Conv. Bioref. 11, 2157–2165 (2021). https://doi.org/10.1007/s13399-020-00605-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00605-4