Abstract
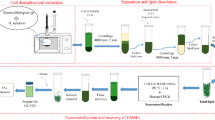
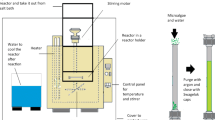
Considerable evidence supports the enhanced utilization of high-quality protein to obtain optimal health subsequences. Microalgae are a valorous source of proteins that can be employed as functional, nutritional, and remedial supplies. An effective microwave-assisted extraction method based on ionic liquids has been utilized in this study to extract proteins from microalgae Nannochloropsis oceanica biomass. The study of microwave-assisted extraction was carried out under varying conditions of temperature (30–80 °C), extraction time (5–35 min), and IL concentration (0–4.67% w/v). Among the six types of examined IL, choline acetate was an effective one for extracting total protein from microalgae biomass. The highest protein yield was obtained at 0.5 g microalgae, 2% w/v [Ch][Ac], and 40 °C for 30 min, representing 26.35% of protein yield and 65.06% of total protein extracted from the Nannochloropsis oceanica biomass. Meanwhile, conventional method of Soxhlet resulted in the protein yield of 0.63%. Only 1.56% of total Nannochloropsis oceanica protein was extracted by extraction method of Soxhlet which uses the hazardous solvent. The outcomes illustrated the superiority of [Ch][Ac]-based microwave-assisted extraction to conventional methods. This innovative procedure is recommended to have significant usage for the separation of proteins. The outcomes from this study would be beneficial in recognizing and harnessing significant microalgae biochemical from IL-based microwave-assisted extraction process to develop new and improved bioproduct technologies.
Graphical abstract







Similar content being viewed by others
References
Yao CK, Muir JG, Gibson PR (2016) Insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther 43(2):181–196
Cuenca-Sánchez M, Navas-Carrillo D, Orenes-Piñero E (2015) Controversies surrounding high-protein diet intake: satiating effect and kidney and bone health. Adv Nutr 6(3):260–266
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 5(1):34
Soto-Sierra L, Stoykova P, Nikolov ZL (2018) Extraction and fractionation of microalgae-based protein products. Algal Res 36:175–192
Shuba ES, Kifle D (2018) Microalgae to biofuels: ‘promising’ alternative and renewable energy, review. Renew Sustain Energy Rev 81:743–755
Jayakumar S, Bhuyar P, Pugazhendhi A, Rahim MHA, Maniam GP, Govindan N (2021) Effects of light intensity and nutrients on the lipid content of marine microalga (diatom) Amphiprora sp. for promising biodiesel production. Sci. Total Environ. 768:145471
Ananthi V, Brindhadevi K, Pugazhendhi A, Arun A (2021) Impact of abiotic factors on biodiesel production by microalgae. Fuel 284:118962
Whangchai K et al (2021) Synergistic supplementation of organic carbon substrates for upgrading neutral lipids and fatty acids contents in microalgae. J Environ Chem Eng 9(4)105482
Halim R, Gladman B, Danquah MK, Webley PA (2011) Oil extraction from microalgae for biodiesel production. Bioresour Technol 102(1):178–185
Zhang Y, Ward V, Dennis D, Plechkova N, Armenta R, Rehmann L (2018) Efficient extraction of a docosahexaenoic acid (DHA)-rich lipid fraction from Thraustochytrium sp. using ionic liquids. Materials (Basel) 11(10):1986
Pérez-Palacios T, Melo A, Cunha S, Ferreira I (2014) Determination of free amino acids in coated foods by GC–MS: optimization of the extraction procedure by using statistical design. Food Anal Methods 7(1):172–180
Lammens TM, Franssen MCR, Scott EL, Sanders JPM (2012) Availability of protein-derived amino acids as feedstock for the production of bio-based chemicals. Biomass Bioenergy 44:168–181
Mitra M, Mishra S (2019) A comparative analysis of different extraction solvent systems on the extractability of eicosapentaenoic acid from the marine eustigmatophyte Nannochloropsisoceanica. Algal Res. 38:101387
Manirafasha E, Ndikubwimana T, Zeng X, Lu Y, Jing K (2016) Phycobiliprotein: potential microalgae derived pharmaceutical and biological reagent. Biochem Eng J 109:282–296
Shah MR et al (2018) Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol 30(1):197–213
Rezaei Motlagh S et al (2021) Ionic liquid-based microwave-assisted extraction of lipid and eicosapentaenoic acid from Nannochloropsis oceanica biomass: experimental optimization approach. J Appl Phycol. https://doi.org/10.1007/s10811-021-02437-9
Zainan NH, Thiruvenkadam S, Danquah MK, Harun R (2019) Biochemical analysis and potential applications of aqueous and solid products generated from subcritical water extraction of microalgae Chlorella pyrenoidosa biomass. J Appl Phycol 1–16
Garoma T, Janda D (2016) Investigation of the effects of microalgal cell concentration and electroporation, microwave and ultrasonication on lipid extraction efficiency. Renew energy 86:117–123
Carullo D et al (2018) Effect of pulsed electric fields and high pressure homogenization on the aqueous extraction of intracellular compounds from the microalgae Chlorella vulgaris. Algal Res 31:60–69
Zhang Y et al (2018) Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew Energy 125:1049–1057
Aliev AM, Abdulagatov IM (2017) The study of microalgae Nannochloropsis salina fatty acid composition of the extracts using different techniques. SCF vs conventional extraction. J Mol Liq 239:96–100
Wahidin S, Idris A, Shaleh SRM (2014) Rapid biodiesel production using wet microalgae via microwave irradiation. Energy Convers Manag 84:227–233
Rezaei Motlagh S et al (2020) Prediction of potential ionic liquids (ILs) for the solid–liquid extraction of docosahexaenoic acid (DHA) from microalgae using COSMO-RS screening model. Biomolecules 10(8)1149
Pan J et al (2016) Microwave-assisted extraction of lipids from microalgae using an ionic liquid solvent [BMIM][HSO4]. Fuel 178:49–55
Krishnan S et al (2020) Microwave-assisted lipid extraction from Chlorella vulgaris in water with 0.5%–2.5% of imidazolium based ionic liquid as additive. Renew Energy 149:244–252. https://doi.org/10.1016/j.renene.2019.12.063
Wahidin S, Idris A, Yusof NM, Kamis NHH, Shaleh SRM (2018) Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energy Convers Manag 171:1397–1404
Fan Y, Niu Z, Xu C, Yang L, Chen F, Zhang H (2019) Biocompatible protic ionic liquids-based microwave-assisted liquid-solid extraction of astaxanthin from Haematococcuspluvialis. Ind. Crops Prod. 141:111809
Rodrigues RDP, Silva ASE, Carlos TAV, Bastos AKP, de Santiago-Aguiar RS, Rocha MVP (2020) Application of protic ionic liquids in the microwave-assisted extraction of phycobiliproteins from Arthrospira platensis with antioxidant activity. Sep. Purif. Technol. 252:117448
McQueen L, Lai D (2019) Ionic liquid aqueous two-phase systems from a pharmaceutical perspective. Front Chem 7:135
Zeng Q et al (2013) Extraction of proteins with ionic liquid aqueous two-phase system based on guanidine ionic liquid. Talanta 116:409–416
Rodrigues RDP, de Castro FC, de Santiago-Aguiar RS, Rocha MVP (2018) Ultrasound-assisted extraction of phycobiliproteins from Spirulina (Arthrospira) platensis using protic ionic liquids as solvent. Algal Res 31:454–462
Martins M et al (2016) Recovery of phycobiliproteins from the red macroalga Gracilaria sp. using ionic liquid aqueous solutions. Green Chem 18(15):4287–4296
Chang Y-K, Show P-L, Lan JC-W, Tsai J-C, Huang C-R (2018) Isolation of C-phycocyanin from Spirulina platensis microalga using ionic liquid based aqueous two-phase system. Bioresour Technol 270:320–327
Rodrigues RDP, de Lima PF, de Santiago-Aguiar RS, Rocha MVP (2019) Evaluation of protic ionic liquids as potential solvents for the heating extraction of phycobiliproteins from Spirulina (Arthrospira) platensis. Algal Res. 38:101391
Ninomiya K, Ogino C, Ishizaki M, Yasuda M, Shimizu N, Takahashi K (Nov. 2015) Effect of post-pretreatment washing on saccharification and co-fermentation from bagasse pretreated with biocompatible cholinium ionic liquid. Biochem Eng J 103:198–204. https://doi.org/10.1016/j.bej.2015.08.002
Chronakis IS (2001) Gelation of edible blue-green algae protein isolate (Spirulina platensis strain pacifica): thermal transitions, rheological properties, and molecular forces involved. J Agric Food Chem 49(2):888–898
Choi S-A, Oh Y-K, Jeong M-J, Kim SW, Lee J-S, Park J-Y (2014) Effects of ionic liquid mixtures on lipid extraction from Chlorella vulgaris. Renew Energy 65:169–174
Viboud S, Papaiconomou N, Cortesi A, Chatel G, Draye M, Fontvieille D (2012) Correlating the structure and composition of ionic liquids with their toxicity on Vibrio fischeri: a systematic study. J Hazard Mater 215:40–48
Liu H, Zhang X, Chen C, Du S, Dong Y (2015) Effects of imidazolium chloride ionic liquids and their toxicity to Scenedesmus obliquus. Ecotoxicol Environ Saf 122:83–90
Pandey S et al (2013) Ionic liquids containing fluorinated β-diketonate anions: synthesis, characterization and potential applications. New J Chem 37(4):909–919
Zhang Q, Benoit M, De Oliveira Vigier K, Barrault J, Jérôme F (2012) Green and inexpensive choline derived solvents for cellulose decrystallization. Chem Eur J 18(4):1043–1046
Sekar S, Surianarayanan M, Ranganathan V, MacFarlane DR, Mandal AB (2012) Choline-based ionic liquids-enhanced biodegradation of azo dyes. Environ Sci Technol 46(9):4902–4908
Gadilohar BL, Shankarling GS (2017) Choline based ionic liquids and their applications in organic transformation. J Mol Liq 227:234–261
Ben Ghanem O et al (2015) Effect of imidazolium-based ionic liquids on bacterial growth inhibition investigated via experimental and QSAR modelling studies. J Hazard Mater 297:198–206
Khan AS, Ibrahim TH, Jabbar NA, Khamis MI, Nancarrow P, Mjalli FS (2021) Ionic liquids and deep eutectic solvents for the recovery of phenolic compounds: effect of ionic liquids structure and process parameters. RSC Adv 11(20):12398–12422
Tsubaki S, Onda A, Hiraoka M, Fujii S, Azuma J, Wada Y (2017) Microwave-assisted water extraction of carbohydrates from unutilized biomass. In: Water extraction of bioactive compounds. Elsevier, pp 199–219
Guo Z, Chen F, Yang H, Liu K, Zhang L (Aug. 2015) Kinetics of protein extraction in reverse micelle. Int J Food Prop 18(8):1707–1718. https://doi.org/10.1080/10942912.2014.919318
Alam MA et al (2021) Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J Clean Prod 127445
Choi IL, Choi SJ, Chun JK, Moon TW (2006) Extraction yield of soluble protein and microstructure of soybean affected by microwave heating. J food Process Preserv 30(4):407–419
Pei Y, Wang J, Wu K, Xuan X, Lu X (2009) Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep Purif Technol 64(3):288–295
Cavonius LR (2016) Fractionation of lipids and proteins from the microalga Nannochloropsis oculata. Res Chalmers se
Varghese T, Pare A (2019) Effect of microwave assisted extraction on yield and protein characteristics of soymilk. J Food Eng 262:92–99
Phongthai S, Lim S-T, Rawdkuen S (2016) Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J Cereal Sci 70:146–154
Rombouts I et al (2020) Food protein network formation and gelation induced by conductive or microwave heating: a focus on hen egg white. Innov Food Sci Emerg Technol 66:102484
Zghaibi N, Omar R, Kamal SMM, Biak DRA, Harun R (2019) Microwave-assisted brine extraction for enhancement of the quantity and quality of lipid production from microalgae Nannochloropsissp. Molecules 24(19):3581
Chew KW, Chia SR, Lee SY, Zhu L, Show PL (2019) Enhanced microalgal protein extraction and purification using sustainable microwave-assisted multiphase partitioning technique. Chem Eng J 367:1–8
Park W-J, Ahn J-H, Hwang S, Lee C-K (2010) Effect of output power, target temperature, and solid concentration on the solubilization of waste activated sludge using microwave irradiation. Bioresour Technol 101(1):S13–S16
Sólyom K, Mato RB, Pérez-Elvira SI, Cocero MJ (2011) The influence of the energy absorbed from microwave pretreatment on biogas production from secondary wastewater sludge. Bioresour Technol 102(23):10849–10854
Li J et al (2010) Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov Food Sci Emerg Technol 11(4):637–643
Zhu G, Zhu X, Fan Q, Wan X (2011) Kinetics of amino acid production from bean dregs by hydrolysis in sub-critical water. Amino Acids 40(4):1107–1113
Zainan NH et al (2019) Kinetic and thermodynamic characterization of amino acids generation via subcritical water reaction of microalgae Nannochloropsis sp. biomass. Biomass Convers Biorefinery 1–14
Chew KW, Ling TC, Show PL (2019) Recent developments and applications of three-phase partitioning for the recovery of proteins. Sep Purif Rev 48(1):52–64
Zghaibi N, Omar R, Kamal SMM, Biak DRA, Harun R (2019) Microwave-assisted brine extraction for enhancement of the quantity and quality of lipid production from microalgae Nannochloropsis sp. Molecules 24:19. https://doi.org/10.3390/molecules24193581
Patil PD, Yadav GD (2018) Application of microwave assisted three phase partitioning method for purification of laccase from Trametes hirsuta. Process Biochem 65:220–227
Chia SR, Chew KW, Zaid HFM, Chu D-T, Tao Y, Show PL (2019) Microalgal protein extraction from Chlorella vulgaris FSP-E using triphasic partitioning technique with sonication. Front Bioeng Biotechnol 7:396
Acknowledgements
The authors are thankful for the University Putra Malaysia (UPM) support for providing the equipment and research facilities to perform this project. The author also would like to acknowledge the International Institute for Halal Research and Training (INHART), International Islamic University Malaysia (IIUM), to provide the ionic liquids for this study. This study was financially supported by Research Management Centre, IIUM, grant no. RMCG20-021-0021.
Author information
Authors and Affiliations
Contributions
Writing—original draft preparation, methodology, and analysis were by S.R.M.; supervision and editing were by A.A.E., R.H., and R.O.; English proof reading by A.A.E. and R.K.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Motlagh, S.R., Elgharbawy, A.A., Khezri, R. et al. Ionic liquid-based microwave-assisted extraction of protein from Nannochloropsis sp. biomass. Biomass Conv. Bioref. 13, 8327–8338 (2023). https://doi.org/10.1007/s13399-021-01778-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01778-2